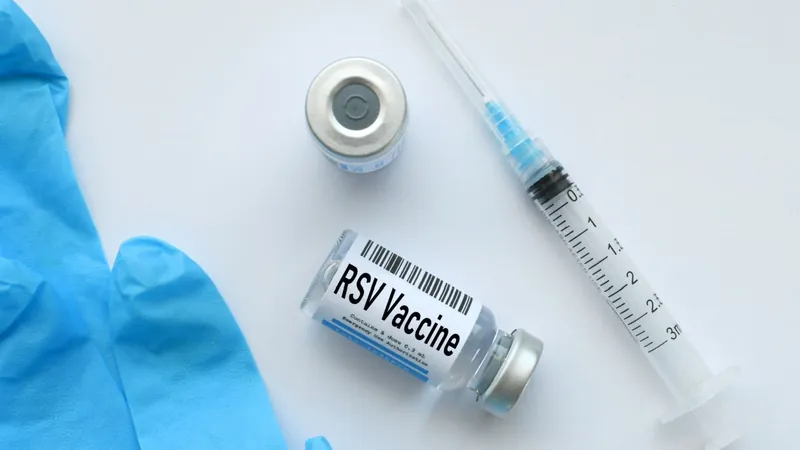
Game Changer: New RSV Vaccine Recommended for Adults Aged 50 to 59!
2025-04-17
Author: Siti
ACIP's Bold Move for RSV Vaccination
In a groundbreaking decision, the US Advisory Committee on Immunization Practices (ACIP) has officially endorsed the use of respiratory syncytial virus (RSV) vaccines, particularly GSK's innovative adjuvanted Arexvy, for adults aged 50 to 59 who are at heightened risk for severe disease.
Who Benefits? The High-Risk Group
This recommendation specifically targets individuals grappling with asthma, heart disease, diabetes, chronic obstructive pulmonary disease (COPD), and those residing in healthcare facilities. Such individuals are significantly more vulnerable to the harsh consequences of RSV, which can escalate to hospitalization, pneumonia, and even death.
Building on Previous Endorsements
This latest recommendation follows ACIP's June 2024 approval for RSV vaccinations for people aged 60 to 74 at high risk, as well as all individuals aged 75 and older. The proactive approach aims to extend immunity to a broader demographic.
Encouraging Trial Results
ACIP's decision was profoundly influenced by promising results from a Phase III trial that evaluated the safety and immune response of the RSV vaccine among those aged 50 to 59. The study highlighted the vaccine’s effectiveness, particularly in individuals with significant health risks associated with RSV lower respiratory tract disease (RSV-LRTD). In earlier trials, a single dose has proven effective for those aged 60 and above.
Next Steps for Healthcare Providers
Once the recommendations receive final approval, healthcare providers will have clear guidelines on how to administer the vaccine, paving the way for insurance coverage decisions that will make this life-saving vaccine accessible to many.
GSK's Excitement Over Expansion of Immunization
Tony Wood, GSK’s Chief Scientific Officer, expressed enthusiasm over the ACIP's recommendation, stating, "We are thrilled to extend the benefits of RSV immunization to over 13 million adults aged 50 to 59 who face severe risks from this virus. RSV significantly impacts those with underlying health conditions, and we’re committed to safeguarding more lives through vaccination."
What Makes Arexvy Unique?
The adjuvanted RSV vaccine fuses recombinant RSV glycoprotein F, stabilized in its prefusion state (RSVPreF3), with GSK's AS01E adjuvant. This innovative approach has already gained approval in 61 countries, including the US, Japan, and across Europe, as a preventive measure against RSV-LRTD for people aged 60 and older.
As RSV poses an increasing challenge, these steps represent a vital advancement in public health aimed at protecting vulnerable populations. This is a story we're watching closely!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)