Game-Changer in Stem Cell Research: New Insights into Mitochondrial Regulation!
2025-04-16
Author: Mei
Unlocking the Secrets of Mitochondria in Stem Cells!
Mitochondria, often dubbed the powerhouses of the cell, are gaining fame for their crucial role not only in energy production but also in the destiny of stem cells. A groundbreaking study has revealed a unique way these organelles communicate with the cell nucleus, significantly impacting how stem cells acquire pluripotency and their eventual fate.
What is UPRmt and Why is it Important?
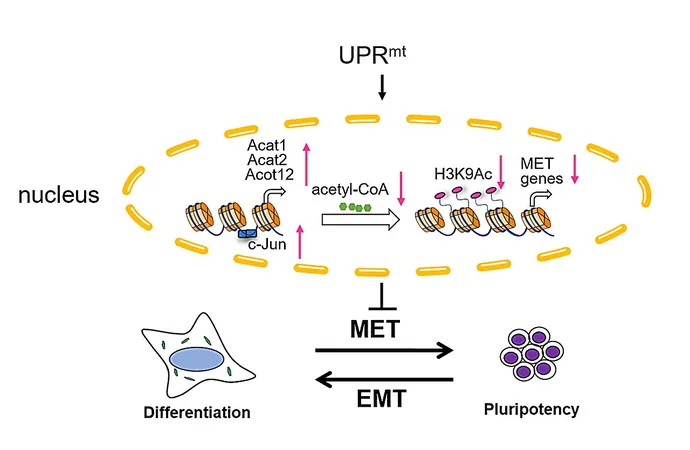
Enter the mitochondrial unfolded protein response (UPRmt)—a vital pathway that acts like a messenger, relaying critical information from mitochondria to the nucleus. Factors such as protein imbalances and heightened levels of reactive oxygen species (ROS) can ignite this pathway, influencing essential gene expression that dictates whether cells will thrive or suffer.
The Research Breakthrough!
Led by Prof. Liu Xingguo from the Guangzhou Institutes of Biomedicine and Health, this influential study, published in *Nature Metabolism*, unveils that UPRmt is transiently activated when cells are on the brink of becoming pluripotent, but this activation fades over time.
Meet c-Myc: The Key Player!
Interestingly, the research pinpointed c-Myc as a pivotal factor in igniting UPRmt. When overexpressed, c-Myc dramatically boosts the levels of Hsp60, a known UPRmt marker, unlike other transcription factors like Sox2 and Klf4 that showed little to no effect.
Impact on Epithelial Transition and Cancer!
This study also shed light on an intriguing relationship: the activation of UPRmt hinders the mesenchymal-to-epithelial transition (MET) during early development. In cancer cells, this activation can surprisingly enhance their migratory and invasive capabilities, pointing to complex roles in both stem cell dynamics and tumor progression.
c-Jun: A Novel Regulator Unearthed!
Delving deeper, researchers identified c-Jun as a novel player within the UPRmt signaling pathway. This proto-oncogene curtails pluripotency acquisition while UPRmt activation nudges its expression upwards. This interaction decreases acetyl-CoA levels—a critical molecule linked to histone acetylation—leading to reduced gene expression essential for MET.
A New Frontier in Medicine!
Remarkably, when researchers supplemented cultures with acetyl-CoA precursors, they were able to restore lost acetylation levels and gene expression vital for MET. This is the first ever revelation of such a mechanism, boldly suggesting that mitochondrial pathways might regulate not only cell fate but also hold implications for regenerative medicine and cancer therapies.
The Future Awaits!
This research paves the way for revolutionary approaches in understanding cellular behavior and holds promise for future advancements in treating diseases and enhancing regenerative practices, placing mitochondria at the forefront of cell fate research!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)