Game-Changer in Science: New Guidelines for Stem Cell-Based Embryo Models Unveiled!
2025-06-10
Author: Wei
Groundbreaking Recommendations Released
The International Society for Stem Cell Research (ISSCR) Embryo Models Working Group has unveiled transformative recommendations aimed at enhancing oversight of stem cell-based embryo models (SCBEM). These guidelines seek to keep pace with the rapid advancements in this groundbreaking field, ensuring that scientific progress unfolds responsibly.
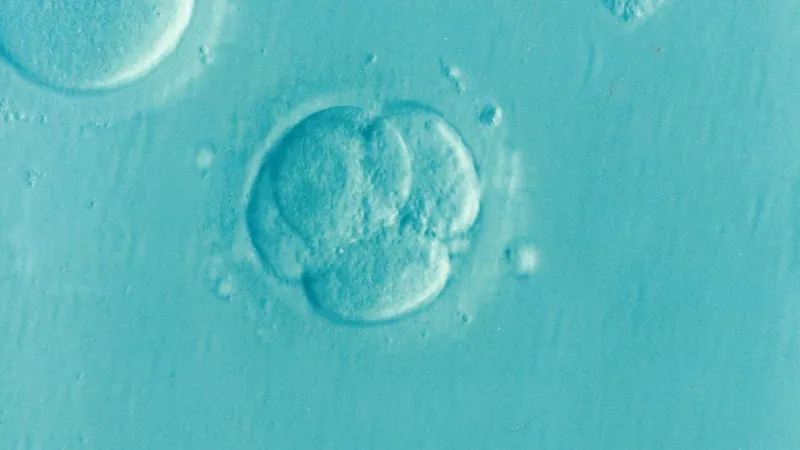
What Are SCBEMs?
Stem cell-based embryo models are innovative three-dimensional structures that mimic crucial elements of early embryonic development. They hold extraordinary potential for deepening our understanding of human life beginnings and improving practices in reproductive science.
Experts Weigh In
Amander Clark, co-chair of the working group and a leading expert at the University of California, Los Angeles, asserts that SCBEMs have the capacity to revolutionize our knowledge of early human development. "The advancements in these models could significantly refine clinical practices in assisted reproduction," she stated.
Clark emphasized the importance of a balanced approach to their study, one that considers ethical, legal, and social concerns alongside scientific innovation. The recently proposed guidelines aim to do just that, providing essential regulatory guidance for key stakeholders in the biomedical research community.
Urgent Need for Updated Guidelines
Janet Rossant, Ph.D., another co-chair and developmental biology expert, highlighted the necessity of these guidelines due to the rapid evolution of technology. "As advancements continue to emerge, we needed to reassess the existing ISSCR Guidelines to ensure they remain relevant and effective," she explained.
Key Recommendations at a Glance
The comprehensive paper titled "Stem cell-based embryo models: the 2021 ISSCR stem cell guidelines revisited" lays out critical recommendations, including:
1. Mandatory review for all research involving three-dimensional SCBEMs.
2. Ensuring all research has a robust scientific rationale.
3. Implementing specific timelines for research progression.
Additionally, the guidelines propose eliminating the distinction between integrated and non-integrated models, clarifying the emerging field and solidifying public trust in the responsible advancement of SCBEM research.
What’s Next?
The proposed recommendations are set to be presented in June to the ISSCR Board of Directors, marking a pivotal update to the 2021 ISSCR Guidelines for Stem Cell Research and Clinical Translation. This move signals an exciting step forward as science, ethics, and public trust converge in a rapidly evolving domain.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)