Explosive New Discoveries: Researchers Unveil Unbelievable Oxygen-Carbon Compounds!
2025-03-27
Author: Mei
Skoltech's Remarkable Breakthrough
In an astounding breakthrough, Skoltech researchers have uncovered a remarkable variety of molecular compounds formed from oxygen and carbon, far beyond familiar substances like carbon dioxide and carbon monoxide.
These groundbreaking oxocarbon compounds are drawing significant attention across multiple fields, including space research, battery technology, biochemical studies, and—surprisingly—industrial explosives and rocket propellants.
Publication and Findings
Published in the esteemed journal *Materials Today Energy*, the study reveals an astonishing array of molecules, with some possessing up to 81% of the explosive energy of TNT.
High-energy-density materials, capable of discharging immense amounts of chemical energy per unit mass, are crucial either as propellants or explosives.
Scientists are eager to discover compounds that could outperform traditional nitrogen-based explosives and rocket fuels, such as ammonium perchlorate.
Exploration of New Chemistry
In their quest, the Skoltech team explored a new chemistry devoid of nitrogen while focusing on oxocarbons.
Lead author and MSc student Elizaveta Vaneeva remarked, "While nitrogen chemistry has dominated high-energy explosives, we hypothesized that carbon oxides might yield even more energetic compounds. To our excitement, we found oxocarbon molecules that release energy exceeding 75% of that of TNT when they decompose into products that include carbon dioxide."
The Molecular Zoo
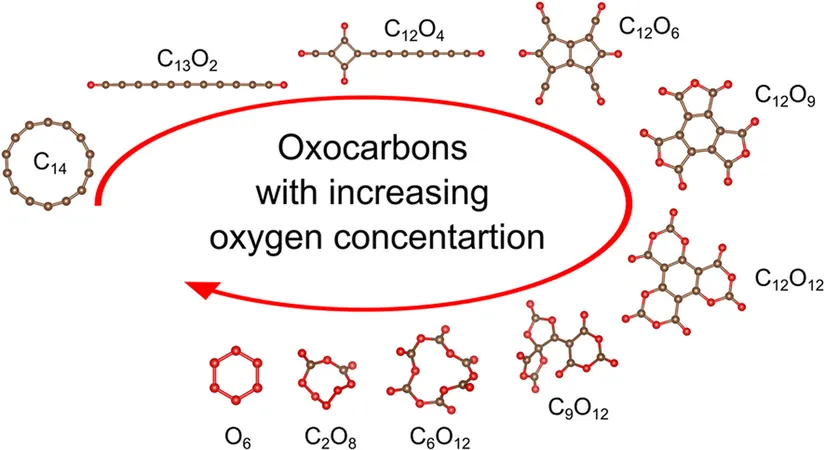
The researchers identified an astounding "molecular zoo" containing 224 oxocarbon compounds, of which only 78 had been previously documented.
Of these, 32 were flagged as potential explosives with real avenues for synthesis.
Among them, compounds such as C₄O₈ and C₄O₉ stand out, alongside the newly discovered C₆O₁₂ and C₆O₁₃, all showcasing 75% or more of TNT's explosive energy, with C₄O₉ being the most potent at a staggering 81%.
Revolutionary Implications
Principal investigator Professor Artem R. Oganov, head of the Material Discovery Laboratory at Skoltech, passionately emphasized the revolutionary nature of this work: "Our findings reveal a remarkable breadth of molecular chemistry—not only providing insights into how these compounds could be utilized but also reshaping our understanding of molecular stability itself."
Methodology and Insights
This striking molecular diversity stands in contrast to the often limited chemical compositions present in crystal structures.
Oganov explained their methodology using "magicity," an idea that transposes the magic numbers known from nuclear physics to the molecular realm.
This innovative approach allows researchers to compare a molecule's energy to its neighboring molecular configurations, giving insights into which compounds are more feasible for formation in nature.
Potential Applications
The potential applications for these oxocarbon compounds are vast, extending into advanced explosive technologies, lithium-ion battery electrodes, and the field of atmospheric chemistry.
They also play a significant role in investigating the combustion products of common fuels like kerosene and ethanol.
Astrophysical Exploration
Moreover, the presence of oxocarbon compounds is expected not just on Earth but also throughout the interstellar medium and on various planets, marking them as a tantalizing area for astrophysical exploration.
Despite the excitement, it's essential to note that many of these carbon-oxygen compounds remain underexplored, with previous literature primarily focusing on well-known molecules like CO₂ and CO.
The Skoltech study marks a significant leap forward in this fascinating domain, paving the way for future research and new discoveries in high-energy materials.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)