Exploring the Impact of Antenatal Corticosteroids on Fetal Growth Restriction: Insights from Animal Studies
2025-03-13
Author: Wei Ling
In a comprehensive systematic review and meta-analysis, researchers have delved deep into the effects of antenatal corticosteroid (CCS) therapy on outcomes in animal models suffering from fetal growth restriction (FGR). Registered under the protocol CRD42022318861 with PROSPERO and conducted following PRISMA guidelines, this study scrutinizes literature to uncover the potential benefits and risks associated with CCS treatment, which is widely used to enhance lung maturation and overall fetal health, especially in preterm births.
Comprehensive Search and Analysis Strategy
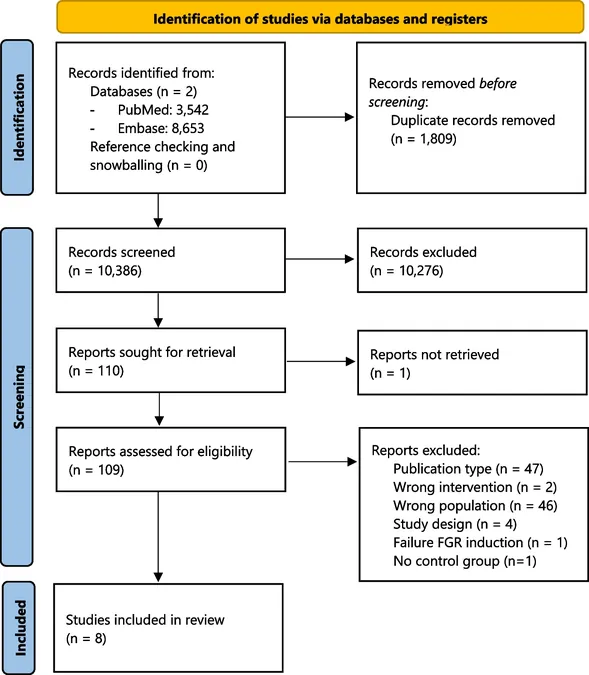
On April 15, 2022, an exhaustive search of the PubMed and Embase databases was conducted, yielding an astounding 10,386 unique articles on the impact of CCS on offspring outcomes in the context of FGR. After a rigorous screening process, only eight studies met the criteria for inclusion, emphasizing strict eligibility based on study design, control group comparability, and proper administration of treatment.
Key Findings on Fetal Development
The analysis involved various species, predominantly sheep, but also included rats and guinea pigs. The study explored multiple parameters such as fetal weight, brain development, and long-term outcomes. Notably, the administration of CCS often resulted in improved lung development across studies, showcasing its beneficial role in promoting respiratory health in FGR offspring.
- Lung Development: Significant advancements in lung morphology and surfactant production were observed, underscoring the effectiveness of CCS in accelerating fetal lung maturation.
- Fetal Weight Impact: The results diverged depending on species; while sheep exhibited a significant reduction in fetal weight following CCS administration, guinea pigs did not show the same adverse effects, arguably due to inherent differences in glucocorticoid receptor affinities among species.
Assessing Neurological and Cardiac Outcomes
The research also investigated the implications of CCS on brain health and cardiac function. While there were alarming indicators of potential brain injury linked to oxidative stress in FGR sheep, the consensus tentatively supports the idea that CCS may not severely compromise neurological development when appropriately managed. Furthermore, no adverse effects on cardiac outcomes or glucose metabolism were identified, suggesting a lack of harmful repercussions from CCS treatment.
Future Implications and Recommendations
The systematic review concludes that though antenatal CCS demonstrates therapeutic benefits akin to those seen in non-FGR subjects, caution is warranted regarding its association with increased brain injury markers. The authors call for further studies in human populations to ascertain the safety and efficacy of CCS, particularly in FGR pregnancies, emphasizing the necessity for meticulous reporting in future research to clarify risk factors and outcomes.
In light of these findings, the research supports the continued use of antenatal CCS in clinical settings where preterm birth is anticipated, specifically for at-risk populations suffering from FGR. However, as science advances, ongoing debate surrounding optimal treatment protocols will undoubtedly continue to shape maternal-fetal medicine practices.
Final Thoughts
As health professionals strive to make informed decisions regarding the care of FGR pregnancies, the importance of evidence-based research remains paramount. This significant analysis serves as a crucial stepping stone toward more tailored therapeutic strategies, ensuring the best possible outcomes for both mothers and their newborns. Stay tuned as we continue to monitor developments in antenatal treatments and their implications in the ever-evolving field of obstetrics!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)