Evaluating Liver Fibrosis and Cirrhosis in COVID-19 Patients: The Role of FIB-4, APRI, and GPR
2025-03-20
Author: Jia
The COVID-19 pandemic caused by SARS-CoV-2 has posed a significant threat to global health, with over 607 million confirmed cases and 6.5 million deaths reported by September 2022. While the primary impact of COVID-19 is respiratory, emerging evidence indicates that the virus can also cause liver damage through both direct and indirect mechanisms.
Research has shown that SARS-CoV-2 can induce liver injury through a cytokine storm—an immune response characterized by excessive inflammation. Studies have revealed that patients with severe COVID-19 often present with elevated levels of inflammatory markers such as IL-6, IL-2, C-reactive protein (CRP), and serum ferritin. Autopsies have indicated T-cell hyperactivation in the liver and associated conditions like microvesicular steatosis, which support the theory of immune-mediated liver damage.
Further aggravation of liver conditions can occur through indirect means, particularly through vascular complications like thrombosis. Many COVID-19 patients have exhibited coagulopathy, affecting liver perfusion. Additionally, some medications used in treating COVID-19, such as antiviral drugs, are known to potentially cause liver toxicity, complicating the clinical picture.
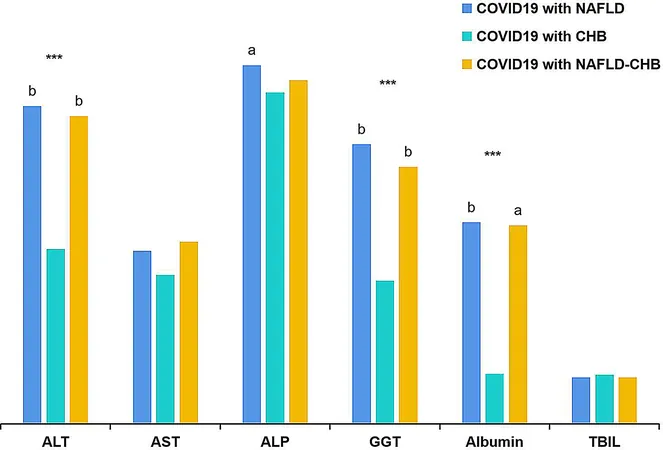
A substantial body of research suggests that pre-existing liver diseases significantly worsen the prognosis of COVID-19 patients. For instance, a study found that chronic liver disease was an independent risk factor for poor outcomes in hospitalized patients. Histological evidence indicates that patients with coexisting conditions like non-alcoholic fatty liver disease (NAFLD) and chronic hepatitis B (CHB) faced higher risks of liver injury and severe COVID-19.
To accurately assess liver fibrosis and cirrhosis in these patients, non-invasive methods such as the FIB-4 index, APRI (aspartate aminotransferase to platelet ratio index), and GPR (gamma-glutamyl transpeptidase to platelet ratio) are essential. Each of these indices has distinct strengths and varies in diagnostic performance. For example, FIB-4 is widely studied and cost-effective, while GPR has shown superior performance in predicting significant fibrosis and cirrhosis in chronic hepatitis B populations.
Despite the advantages of these non-invasive methods, limited research exists on their effectiveness for COVID-19 patients with concurrent liver diseases. Studies focusing on overall mortality have mostly excluded patients with chronic liver conditions, creating a gap in understanding how to effectively evaluate liver fibrosis in this specific demographic.
This study involved 744 hospitalized COVID-19 patients, examining the consistency and diagnostic capability of the FIB-4, APRI, and GPR in assessing liver fibrosis levels. Results showed reasonable concordance among these methods when assessing significant liver fibrosis, suggesting they can effectively help identify at-risk patients without requiring invasive procedures.
Inclusion criteria for the study ensured a comprehensive evaluation of patients aged 18 to 65, diagnosed with COVID-19 and liver diseases, such as NAFLD and CHB. Statistical analyses conducted included logistic regression and chi-square tests, ensuring rigorous data examination.
Findings indicated that COVID-19 patients with higher fibrosis scores showed a greater incidence of severe disease and extended time to viral clearance. This correlation emphasizes the relationship between liver health and COVID-19 outcomes, potentially guiding clinical management strategies.
In conclusion, the FIB-4, APRI, and GPR indices represent valuable tools for assessing liver fibrosis in COVID-19 patients with concurrent liver diseases. While non-invasive assessments provide critical insights, continued research is needed to optimize their clinical applications and validate their diagnostic capabilities against liver biopsy—the current gold standard. Moving forward, studies should aim to fill existing gaps by establishing more comprehensive evaluative frameworks for this vulnerable patient population.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)