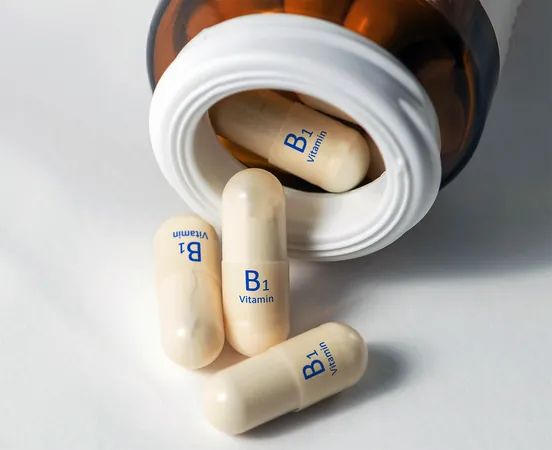
Eureka! Groundbreaking Vitamin B1 Study Proves 1958 Theory After Decades of Doubt
2025-08-30
Author: John Tan
A Chemistry Breakthrough Years in the Making
For generations, a fundamental chemistry lesson has held that certain high-energy compounds, such as vitamin B1, simply couldn't survive in water. This belief forced many chemical reactions to be conducted in specialized organic solvents instead. But a groundbreaking new study shatters that long-standing rule, revealing that a reactive carbon species can not only exist in water but can also be clearly observed.
The Bold Vitamin B1 Theory of 1958
The controversy traces back to 1958 when scientists proposed that vitamin B1 could create a fleeting, carbene-like species within living cells. This idea was met with skepticism because it contradicted the established norm that water would destroy carbenes almost instantly. Over the decades, as analytical tools advanced, researchers welcomed the challenge of finding direct evidence.
Proof at Last: The Study That Changed Everything
Recently, researchers achieved this monumental proof by ingeniously designing a molecule that protects the reactive center well enough to remain stable in liquid water. "This is the first time anyone has been able to observe a stable carbene in water," declared Vincent Lavallo, a chemistry professor at UC Riverside, emphasizing the significance of this discovery.
Understanding Carbenes and Their Significance
Carbenes are reactive intermediates featuring a carbon atom with two unfilled bonding spots, making them highly reactive. This property is advantageous in various lab and industrial reactions, often rearranging bonds with great efficiency. Traditionally, chemists believed that water molecules would prematurely quench these carbenes, blocking their potential role in biological systems.
How They Did It: An Innovative Approach
Innovatively, the research team surrounded the carbene with bulky molecular groups to prevent water from interfering. This strategic crowding allowed them to generate the carbene in water and capture its signature using nuclear magnetic resonance (NMR) spectroscopy, providing robust evidence of its existence.
Connecting the Dots: Vitamin B1 and Clean Chemistry
Vitamin B1, or thiamine, is essential for numerous metabolic processes in the body, facilitating crucial carbon-carbon bond transformations. The 1958 proposal suggested that a carbene-like state formed by vitamin B1 could help catalyze such reactions, and this recent study moves forward this intriguing concept. Essentially, it opens the door to using safer, environmentally-friendly water as a solvent for chemical reactions.
A Greener Future for Chemistry
"Water is the ideal solvent—it's abundant, non-toxic, and environmentally friendly," stated Varun Raviprolu, the first author of the study. The potential shift towards utilizing water in chemical manufacturing could dramatically reduce the risks associated with traditional organic solvents, paving the way for safer production processes.
The Exciting Implications Ahead
This discovery is just the beginning. "There are other reactive intermediates we've never been able to isolate, much like this one," Lavallo remarked. By employing protective strategies, scientists may eventually capture other elusive chemical species that play vital roles in various chemical reactions.
A Historic Confirmation with Modern Relevance
While this study does not capture vitamin B1 carbene formation inside living cells in real time, it effectively affirms a long-debated concept that water does not automatically preclude carbene chemistry. This aligns with historical scientific theory while pointing towards innovative and eco-friendly ways to conduct chemical reactions.
As Lavallo noted, "Just 30 years ago, people thought these molecules couldn’t even be made. Now, we can bottle them in water. What Breslow proposed all those years ago—he was right." This remarkable study highlights how science evolves, confirming ideas long dismissed as impossible.
Conclusion: The Future of Chemistry is Bright
This meticulous piece of research not only marks a milestone in understanding how vitamin B1 functions but also serves as a powerful reminder that impactful ideas can take decades to be validated. The full study is now available in the journal Science Advances, marking a new chapter in chemical research.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)