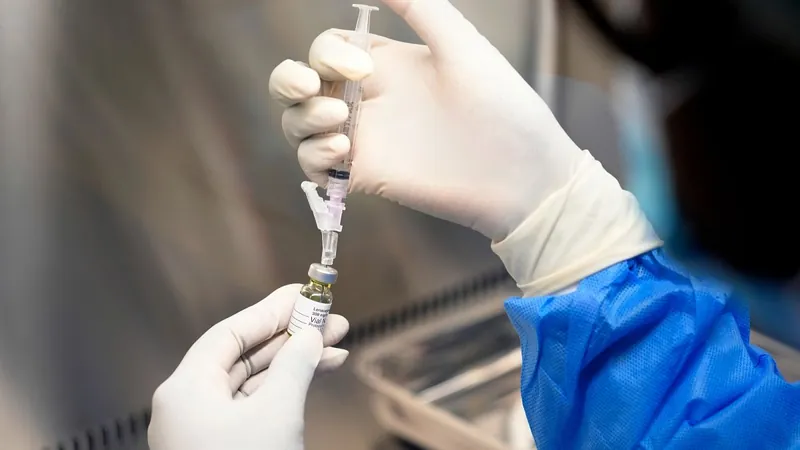
EU Approves Revolutionary Twice-Yearly HIV Injection: A Game-Changer in Prevention!
2025-07-25
Author: Siti
Groundbreaking Approval for HIV Prevention
In a monumental stride against the HIV epidemic, European regulators have just approved a revolutionary, twice-yearly injection that promises to change the landscape of HIV prevention forever. This groundbreaking jab, developed by Gilead, is hailed as one of the most significant medical breakthroughs of 2024, providing a compelling alternative to the daily pill regimen.
Introducing Lenacapavir: The Future of Pre-Exposure Prophylaxis (PrEP)
Named lenacapavir, this innovative drug demonstrated a staggering 100% effectiveness in clinical trials, marking it as a frontrunner in the realm of pre-exposure prophylaxis (PrEP). It works by halting the replication and spread of HIV within the body, significantly lowering the risk of infection among both adults and adolescents.
A New Era of HIV Prevention in Europe
Following a positive recommendation from the European Medicines Agency's advisory committee, the path is now clear for the European Commission to grant official approval in the coming months. Dr. Dietmar Berger, Gilead Sciences’ chief medical officer, expressed enthusiasm, stating, "This milestone reflects our commitment to reimagine HIV prevention in Europe and around the world. Lenacapavir for PrEP has the potential to become a critical tool for public health, particularly for those facing the highest barriers to care."
Addressing the Rise in HIV Cases
Despite advancements in HIV treatment and prevention, alarming trends continue. In 2023, over 24,700 new HIV diagnoses were reported across the European Union, Iceland, Liechtenstein, and Norway—a worrying 11.8% increase from the previous year. This highlights the urgent need for innovative solutions like lenacapavir.
Global Reach and Accessibility
The U.S. Food and Drug Administration (FDA) previously approved lenacapavir last month, with Gilead planning to market it as Yeytuo in Europe. Notably, Gilead has committed to providing generic versions of the drug in 120 lower-income countries that experience high rates of HIV, although the exact availability of these generics remains in question, especially following funding cuts to global health initiatives in the U.S.
A Hopeful Future for HIV Prevention
As the world grapples with the complexities of HIV, the introduction of lenacapavir stands as a beacon of hope. This new approach to prevention not only aims to reduce infection rates but also to empower individuals, providing them with more options for safeguarding their health in the ongoing battle against HIV.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)