Deadly Bacteria Unveil Secrets to Sneak Into Your Cells Using Sugar Signals
2025-05-12
Author: Wei
Groundbreaking Discovery on Bacterial Infections
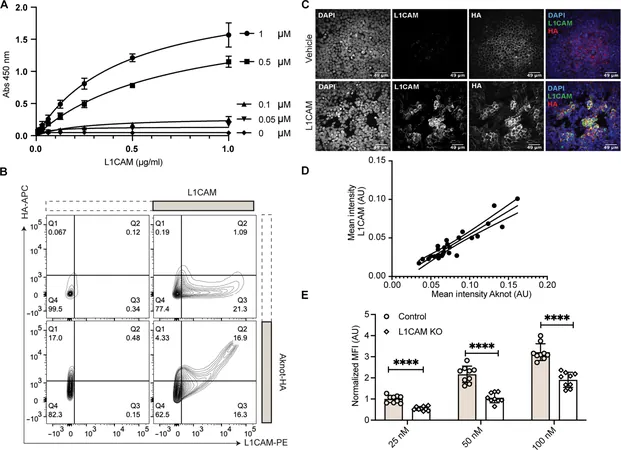
In a stunning revelation, researchers led by Dr. Karla Satchell from Northwestern University have unearthed a crucial insight into how lethal bacteria, particularly the notorious Vibrio vulnificus, invade human cells. This astonishing study, recently published in *Science Advances*, sheds light on the sugars that act as vital signals in the infection process.
The Role of MARTX Toxins in Cell Invasion
Known as multifunctional autoprocessing repeats-in-toxin (MARTX) toxins, these secreted agents are pivotal in spreading various Gram-negative bacteria, especially those lurking in raw or undercooked shellfish. Their ability to penetrate host cells and cause severe infections has remained largely enigmatic—until now.
How Do Bacteria Choose Their Victims?
Dr. Satchell and her team set out to unravel this mystery, probing the surface interactions that allow these toxins to identify their preferred targets on human cells. Their innovative techniques led them to discover a specific part of the MARTX toxin that binds directly to sugar molecules on the cell surface.
A Sweet Spot: Sugar Recognition and Infection
The research revealed that MARTX toxins preferentially attach to N-acetylglucosamine (GlcNAc)—a vital sugar component found on epithelial cells—highlighting how different cells have unique sugar signatures. "This divergence in sugar structures is what allows toxins to determine which cell type to attack," Satchell explained.
A Critical Mechanism for Intestinal Infections
Furthermore, the study confirmed that this sugar-binding domain is essential for Vibrio vulnificus to establish infections in the intestine, suggesting a broader implication of MARTX toxins in bacterial pathogenicity.
Implications for Future Research
This groundbreaking finding, articulated by lead author Jiexi Chen, underscores why MARTX toxins are so commonly found in nature—recognizing a structural motif present on various host surfaces can significantly enhance our understanding of bacterial infections. The implications for treatment and prevention are monumental.
As scientists delve deeper into these molecular mechanisms, the possibilities for developing targeted therapies against such deadly pathogens become more promising.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)