CRISPR Breakthrough: Unraveling a Powerful New Antiviral Mechanism!
2024-10-28
Author: Siti
CRISPR-Cas10: A New Antiviral Mechanism
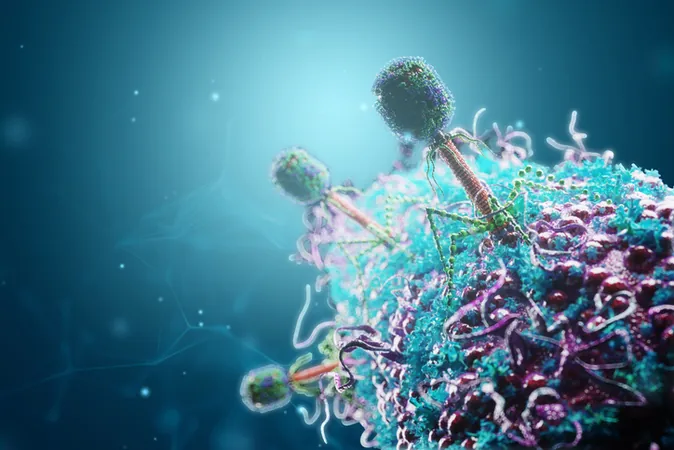
In an astonishing discovery that broadens the understanding of bacterial defenses, researchers have unveiled a new antiviral mechanism employed by the lesser-known CRISPR-Cas10 system, presenting a fascinating contrast to its more famous cousin, CRISPR-Cas9. While CRISPR-Cas9 functions akin to molecular scissors that slice foreign DNA or RNA to protect bacteria from viral attacks, CRISPR-Cas10 takes on a drastically different role, acting more like a molecular fumigator to combat viral infections.
Mechanism of Action
When activated by an attacking virus, CRISPR-Cas10 triggers an intricate cascade of events that culminates in the production of a toxic cloud of inosine triphosphate (ITP). This ITP is synthesized through the deamination of adenosine triphosphate (ATP) and serves to stifle the growth of the infected bacterium, ultimately sacrificing the cell but ensuring the survival of the bacterial community by preventing the proliferation of the phages among neighboring bacteria.
Research Insights
This pivotal research was spearheaded by renowned scientists Dinshaw Patel, PhD, of the Memorial Sloan Kettering Cancer Center, and Luciano Marraffini, PhD, from Rockefeller University, with their findings published in the prestigious journal Cell under the title, 'The CRISPR-associated adenosine deaminase Cad1 converts ATP to ITP to provide antiviral immunity.'
Cas10's Dual Role
In their article, the authors explain the two-fold activity of Cas10: it not only degrades single-stranded DNA, providing immediate anti-phage immunity when viral transcripts are present early in the infection cycle, but also synthesizes cyclic oligoadenylates (cOAs). The cOAs play a critical defensive role, particularly against viruses whose RNA is expressed later during the infection. This groundbreaking study placed a particular focus on cOA synthesis, building upon existing knowledge that linked Cas10's actions to pathways similar to those in mammalian innate immunity that produce cyclic nucleotides.
Discovery of Cad1
A remarkable element of this study was the role of a newly discovered CRISPR protein, known as CRISPR-associated adenosine deaminase 1 (Cad1). The researchers revealed that Cad1, once activated by the cOAs, converts ATP to ITP in both test tube and living systems. Using advanced cryoelectron microscopy, they detailed the structure of Cad1, which forms a hexameric assembly crucial for its activity; this allows it to bind ATP and ITP effectively during the deamination process.
Efficiency and Implications
Co-first author Christian F. Baca described the efficiency of the Cas10 response, noting that while it can neutralize early-stage viruses effectively, those that emerge later require the production of cOAs for defense. This complex interaction showcases the cut-throat battle that occurs at microscopic levels—highlighting how infected bacterial cells may perish in the process, but their larger population survives.
Mysteries of ITP
The toxic nature of ITP to the bacterium remains a mystery, with theories suggesting it competes with ATP and GTP—critical for various cellular functions—or prevents phage DNA replication. As co-first author Puja Majumder articulated, the pathogen’s aggressiveness directly shows the lengths bacteria go to safeguard their kin.
Future Directions
Beyond the fascinating biology revealed, this research carries potential for practical applications. The detection of ITP could serve as an innovative diagnostic tool for pathogens, alerting us to their presence within clinical samples. With their groundbreaking findings, these scientists underscore the diversity of molecular strategies that CRISPR systems employ to impart immunity in prokaryotic organisms—signaling a pivotal moment in the intersection of microbiology and genetic engineering.
Conclusion
Stay tuned for further breakthroughs that continue to reshape our understanding of microbial defenses and could pave the way for novel anti-viral strategies!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)