Breakthrough Supramolecular Framework Revolutionizes Iodine Extraction from Seawater: A Game Changer in Pollution Management!
2025-03-12
Author: Arjun
Iodine is an essential element for numerous industries, from pharmaceuticals to food preservation. However, it remains one of Earth's least abundant nonmetallic elements, with seawater containing around 70% of the global reserves—albeit at a mere concentration of about 60 parts per billion (ppb), making extraction a formidable task. The issue intensifies with the presence of radioactive iodine released during nuclear incidents, posing severe threats to marine ecosystems and human health. There is an urgent demand for sophisticated methods to extract iodine from seawater and tackle radioactive iodine contamination.
In an exciting development, researchers from Hainan University have unveiled a groundbreaking supramolecular organic framework (SOF) capable of efficiently capturing iodine from seawater. This innovative framework has successfully removed 79% of iodine pollution in controlled experiments and boasts an astonishing iodine adsorption capacity of 46 mg of iodine per gram within a 20-day extraction timeframe when tested with natural seawater. Their findings have been published in the esteemed journal Research.
“Our work addresses the pressing need for sustainable iodine extraction, which is critical not just for meeting the escalating global demand but also for reducing the ecological hazards tied to radioactive iodine,” stated Ning Wang, the senior author of the study and a professor at the State Key Laboratory of Marine Resource Utilization in the South China Sea, Hainan University. “Innovative materials like our SOF can significantly enhance the selectivity and efficiency of iodine extraction, paving the way for future advancements in this field.
Exploring the Architecture of the SOF
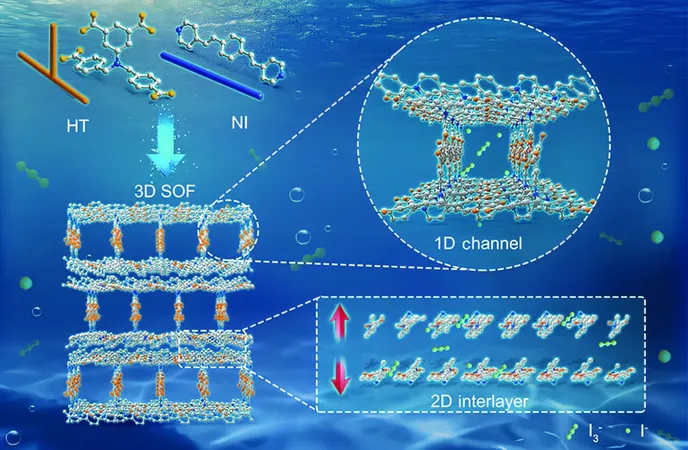
Supramolecular organic frameworks are unique crystalline porous materials characterized by weak noncovalent bonds such as hydrogen bonds and π-π stacking interactions. Their design offers plentiful active sites and adaptable storage capabilities, making them ideal for sequestering iodine. In this study, the researchers engineered a novel three-dimensional (3D) SOF through the self-assembly of two flexible compounds, resulting in interconnected one-dimensional channels and two-dimensional interlayer spaces. This clever architecture provides a heightened iodine adsorption capacity and flexibility.
Exceptional Selectivity and Reusability
The researchers have discovered that this 3D SOF has an extraordinary capacity for triiodide, achieving one of the highest reported iodine adsorption levels in aqueous environments. Remarkably, the material retains over 88% of its initial adsorption performance even after being reused six times, demonstrating its impressive durability. This high-performance SOF showed a selective adsorption proficiency for triiodide in nuclear-contaminated seawater and iodide from natural seawater, thanks to the synergistic effects of amine and pyridyl groups interacting with aromatic rings.
Professor Wang emphasized, “A comprehensive understanding of the chemical and environmental processes involved in extracting iodine from marine ecosystems is essential. The diverse forms of iodine, including iodides and iodates, and their interactions with various ions in seawater underscore the necessity for highly selective and efficient extraction methods. These strategies are crucial for achieving sustainable iodine recovery and long-term reduction of iodine pollution.
This groundbreaking research not only presents a promising solution for iodine extraction but also sets a new benchmark for addressing environmental pollutants. With the potential to revolutionize the industry, these findings signal a brighter future for both iodine resource management and pollution control. Stay tuned for more updates on this exciting development!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)