Breakthrough Study Unveils Key Mechanisms Behind Alzheimer's Protein Clumping
2025-06-11
Author: John Tan
In a significant leap forward in Alzheimer's research, a groundbreaking large-scale study has pinpointed the first molecular events that lead to the formation of harmful amyloid protein aggregates, unveiling a promising new therapeutic target.
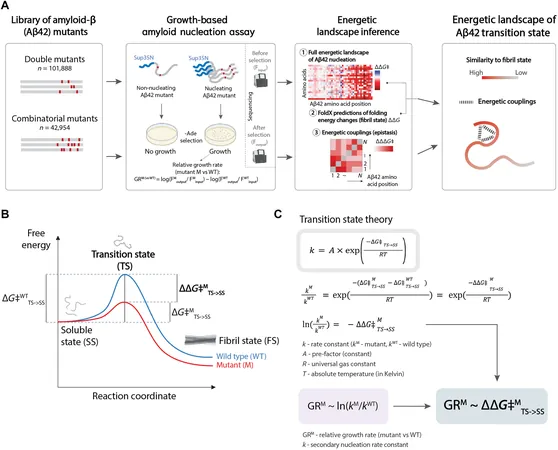
Published in *Science Advances*, a collaboration among the Wellcome Sanger Institute, the Center of Genomic Regulation (CRG), and the Institute for Bioengineering of Catalonia (IBEC) used an innovative blend of genomics and machine learning to explore over 140,000 variants of the amyloid peptide Aβ42—culprits in the development of Alzheimer’s.
With dementia affecting over 55 million people worldwide, and Alzheimer’s being responsible for up to 70% of these cases, this research could redefine our understanding and approach to combating this devastating disease.
The Amyloid Mystery Unlocked
Amyloid beta (Aβ), a peptide comprised of a chain of amino acids, has a notorious reputation for aggregating into structures known as amyloid fibrils, a hallmark of Alzheimer's and more than 50 other neurodegenerative diseases. These fibrils gradually accumulate into toxic plaques, wreaking havoc in the brain.
Crucially, understanding the transition state—the fleeting stage before Aβ begins to form stable fibrils—has been a major challenge, as most individuals don’t experience this process due to its energy demands.
A Pioneering Approach
In their pioneering study, researchers sought to dissect how genetic variations in Aβ42 impact aggregation rates. Their methodology harnessed advanced DNA synthesis techniques along with genetically modified yeast cells, analyzing reaction speeds with the help of machine learning.
This extensive analysis allowed them to simultaneously examine an unprecedented number of Aβ42 variations, vastly enhancing the accuracy and quality of their findings.
Critical Findings
The study revealed that only a few specific interactions within the amyloid protein significantly affect fibril formation speed, underscoring the importance of the C-terminal region of Aβ42. The researchers propose that these interactions are key to preventing the aggregation process that contributes to Alzheimer’s.
Dr. Anna Arutyunyan, a key author of the study, emphasized the study’s groundbreaking nature: "We have created the first comprehensive map illustrating how individual mutations alter the amyloid beta aggregation process. This information is crucial for developing targeted therapeutic strategies."
Future Implications
This innovative study lays the groundwork not just for Alzheimer’s treatments but also for advancing our understanding of protein behaviors across various diseases. Dr. Benedetta Bolognesi highlighted the dual significance of their approach, indicating that it could vastly broaden the horizons for biological research and therapeutic development.
Dr. Richard Oakley from the Alzheimer's Society remarked on the study’s relevance, noting its potential to enhance our understanding of the complex processes that underlie Alzheimer's.
In summary, the research signifies a critical step toward unlocking the secrets behind Alzheimer's and potentially opening doors to more effective treatments. Professor Ben Lehner encapsulated the excitement surrounding this discovery, asserting that this unprecedented scale of analysis signifies a powerful new direction in our quest to combat neurodegenerative diseases.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)