Breakthrough Study Uncovers Genetic Secrets Behind Congenital Heart Disease
2025-03-25
Author: Arjun
Abstract
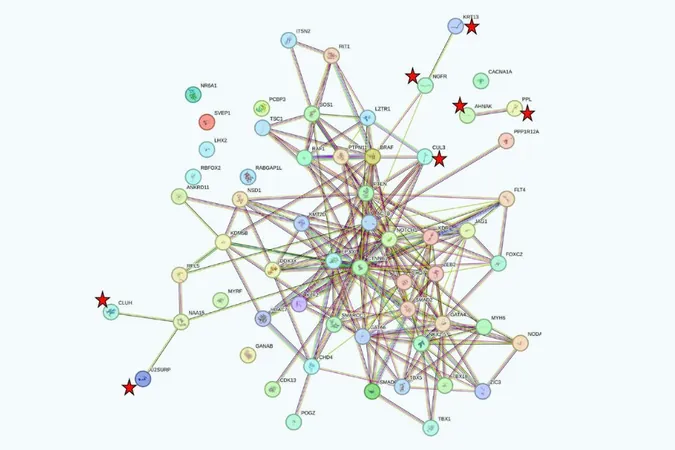
Congenital heart disease (CHD) ranks as one of the most prevalent birth defects, yet its genetic causes have remained largely elusive. A groundbreaking study involving over 11,000 children with CHD has successfully identified 60 genes that are significantly mutated in these patients, far more than would typically occur by chance.
Collaboration and Objectives
This research is part of the Pediatric Cardiac Genomics Consortium, a collaborative initiative spearheaded by the National Heart, Lung and Blood Institute of the NIH. The aim is to unravel the genetic factors contributing to CHD while also examining how these factors relate to clinical features and outcomes in affected individuals.
Key Findings
Published in the Proceedings of the National Academy of Sciences, the study reveals a complex genetic landscape; notably, more than half of the implicated genes are specifically associated with certain heart defects—like the tetralogy of Fallot—while others are linked to a spectrum of CHD subtypes and neurodevelopmental disorders, including autism. The mutations identified arise either spontaneously (de novo) or are inherited from clinically unaffected parents, providing vital insights that could redefine prenatal screening and early risk assessment protocols.
Expert Commentary
Dr. Richard Lifton, the senior author and head of the Laboratory of Human Genetics and Genomics at The Rockefeller University, expressed the surprising nature of the findings: “These results not only enhance our understanding of the intricate biology behind heart development, but they also enable physicians to better screen children for genetic causes of CHD, thereby clarifying diagnoses and predicting risks for future children.”
Historical Context
Historically, previous research by the PCGC indicated that about 9% of CHD cases stem from new genetic mutations. This study now demonstrates that the 60 identified genes account for approximately 60% of this genetic signal. Even more intriguing, about 5% of CHD cases result from genetic mutations passed down by parents who often show no clinical signs of CHD.
Gene Contributions
Among the most notable discoveries in this study is the identification of 10 genes that play crucial roles in chromatin modification, underscoring their significance in gene expression regulation—a concept advanced by the late C. David Allis at Rockefeller University. The frequency of mutations linked to this pathway in CHD, as well as in neurodevelopmental disorders, attests to the impact of Allis's pioneering research.
Inherited Mutations
One of the unexpected revelations was that nearly half of the genetic contributions to CHD stemmed from inherited mutations rather than new mutations. In specific cases, subsequent offspring carried the same mutations and developed CHD, although the degree of risk remains unclear. The incomplete penetrance observed—where parents possess mutations but do not exhibit CHD—suggests that either genetic or environmental factors may further influence disease development.
Gene-Specific Findings
The findings also highlight that while 33 of the identified genes closely correlate with specific CHD subtypes, others are linked to a wider array of cardiac anomalies. For instance, mutations in the NOTCH1 gene are particularly interesting—those mutations that modify critical cysteine amino acids are primarily linked to tetralogy of Fallot and similar defects, while others can result in a broader range of heart diseases. Dr. Lifton noted, “Some genetic mutations reliably cause only one type of heart condition, while others seem unpredictable in their effects.”
Neurodevelopmental Correlations
Furthermore, it's compelling to note that 37 of the identified genes are strongly predictive of neurodevelopmental disorders like autism. Many of these genes have also been implicated in these disorders previously. Collaborating with Rockefeller’s Junyue Cao, the researchers discovered distinct expression patterns among the genes. For example, mutations in MYH6 are primarily linked to cardiac tissue, contrasting with those associated with broader organ implications.
Clinical Implications
The clinical ramifications of these findings are significant. They have the potential to reshape screening protocols for neurodevelopmental disorders. “Early intervention holds promise for improving outcomes in conditions like autism,” explained Brueckner, another key author of the study. Given that CHD is often detectable at birth, doctors could leverage insights from this study to identify high-risk infants in their initial weeks of life, before neurodevelopmental issues emerge, thus maximizing the opportunity for timely intervention.
Routine Genetic Screening
This research emphasizes the increasing relevance of routine genetic screening for patients with CHD. Among those studied, one-third had mutations in genes associated with additional pathologies linked to established syndromes, which were not always diagnosed clinically—often due to the absence of hallmark features of those syndromes. Without genetic testing, children at risk for neurodevelopmental disorders or treatable heart complications, such as arrhythmias, might go unrecognized.
Conclusion
Dr. Lifton remarked on the practical implications of their findings: “Given the complexities of caring for children with severe CHD—who often require surgery and continuous care—the declining cost of comprehensive genetic sequencing represents a minor expense. However, the advantage of early diagnosis is immense, empowering healthcare providers to anticipate issues and hopefully facilitate better health outcomes.” As the understanding of CHD's genetic elements deepens, this research heralds a new chapter in the fight against heart disease, offering hope for affected families and potentially transforming clinical practices.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)