Breakthrough Study Reveals Early Role of Tau Hyperphosphorylation in Neurodegenerative Diseases—What You Need to Know!
2025-01-17
Author: Daniel
Understanding Tau Protein
In the intricate world of neuroscience, tau protein plays a crucial role in maintaining the stability and structure of neurons. This microtubule-associated protein primarily supports microtubules—cylindrical structures vital for cell movement, intracellular transport, and the overall maintenance of a cell’s shape. While tau performs essential neurophysiological functions, its pathological alterations lead to significant concerns as they contribute to various neurodegenerative diseases, collectively known as tauopathies.
The Link Between Tau and Neurodegeneration
The connection between tau accumulation in the brains of individuals with Alzheimer's disease and frontotemporal dementia has been established; higher tau levels correlate with increased neuronal dysfunction and degeneration. However, the detailed processes by which abnormal tau leads to brain toxicity in tauopathies remain largely elusive.
Challenges in Studying Tau Pathology
A significant challenge in uncovering these mechanisms has been the difficulty of studying tau pathology in living humans. Current methodologies often fall short, and effective animal models that accurately replicate the initiation and progression of human tauopathies have been scarce—until now.
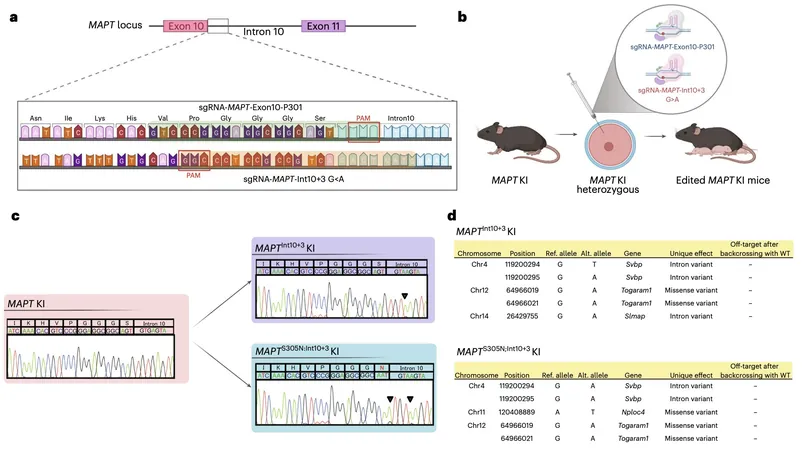
Innovative Mouse Models of Tauopathy
A collaborative effort from esteemed researchers at the RIKEN Center for Brain Science and the UK Dementia Research Institute at University College London has led to the development of innovative genetically modified mouse models. These mice express humanized versions of the MAPT gene, which encodes the tau protein. Their findings, published in Nature Neuroscience, indicate that tau hyperphosphorylation—a process where phosphate groups attach to tau—plays a vital role in the early stages of tau pathology.
Key Findings of the Study
The authors of the study, Naoto Watamura, Martha S. Foiani, and their colleagues, emphasized the need to examine the nuances of tau toxicity, noting the ambiguity regarding the sequence of pathological events and the specific forms of tau responsible for neurotoxicity. Their research introduced targeted mouse lines reflecting mutations linked to frontotemporal dementia, enabling a closer exploration of tau hyperphosphorylation's impact.
Observations from Genetically Modified Models
Through their genetically modified models, the researchers observed an accumulation of hyperphosphorylated tau in the hippocampus and entorhinal cortex, critical areas for memory and cognitive function. They reported that this accumulation occurs without the formation of higher-order fibrillar structures typically associated with advanced tau pathology. Instead, significant neurite degeneration, loss of synapses, and indicators of behavioral abnormalities were recorded, illuminating how tau hyperphosphorylation can instigate early neurodegenerative changes.
Implications of the Research
The implications of these findings are profound. Evidence gathered from this study suggests that neuronal toxicity can manifest independently of fibrillar tau aggregates, proposing that hyperphosphorylation may be one of the earliest contributors to tauopathies, especially those exhibiting isoform ratio imbalances.
Future Directions in Research
Looking ahead, the research team hopes these insights into tau hyperphosphorylation will facilitate further exploration of its link to the onset of Alzheimer’s disease, frontotemporal dementia, and related disorders. Potentially, this could lead to the identification of novel therapeutic targets, fostering strategies that may prevent or retard neurodegeneration in affected populations.
Conclusion
This groundbreaking research paves the way for a deeper understanding of the complex mechanisms underlying neurodegenerative diseases, potentially helping millions grappling with these devastating conditions. Stay tuned as we continue to unravel the mysteries of tau pathology and its implications for future treatments!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)