Breakthrough Study Reveals a Hidden Mechanism Behind Kidney Cancers
2025-06-04
Author: Jia
New Insights into Cancer Mechanisms
In a groundbreaking study from UT Southwestern Medical Center, researchers have unveiled how a genetic mutation that fuses two genes incites various cancer types, including an aggressive form of kidney cancer. This pioneering research, published in the prestigious journal Cell, opens doors to potential new treatments for kidney cancer and may even provide insights for tackling a multitude of other cancers.
Unraveling the Mystery of Oncofusions
Dr. Benjamin Sabari, an Assistant Professor at UT Southwestern and co-lead of the study, stated that the research identifies a shared molecular mechanism across different cancer-driving gene fusions, potentially revealing new vulnerabilities that could be targeted with drugs. The mutations known as chromosomal translocations are present in about 17% of malignancies, yet their exact role in promoting cancer remains uncertain for many types.
The Challenge of Translocation Renal Cell Carcinoma
Specifically, translocation renal cell carcinoma (tRCC) is an exceptionally aggressive subtype, impacting roughly 4% of adult kidney cancer cases and being the most prevalent form in children. Alarmingly, around two-thirds of tRCC cases involve the fusion of the gene for the transcription factor TFE3 with one of three genes—PRCC, ASPL, or SFPQ. What makes this fusion troublesome is that these genes appear unrelated, leaving researchers puzzled about why this juxtaposition occurs.
Revolutionary Findings on Biomolecular Condensates
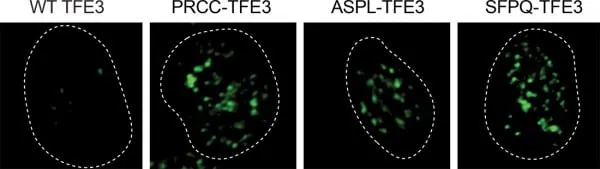
To uncover the mechanisms behind these carcinogenic fusions, Dr. Sabari and his team investigated tRCC cells from patients enrolled in the UTSW Kidney Cancer Program. What they discovered raised eyebrows: the oncofusion proteins formed structures known as biomolecular condensates within cells, while the normal TFE3 protein did not. This suggests a critical role for these condensates in the cancer-promoting process.
A Breakthrough in Understanding Protein Interactions
Previous research by the lab indicated that these condensates could regulate transcription—key for producing proteins from genes—by sequestering essential regulatory proteins. When they mixed TFE3 oncofusion proteins with material extracted from cell nuclei, the result was stunning: these hybrid proteins readily formed condensates, confirming their role in driving cancer.
A Potential Opening for New Treatments
But that’s not all. By comparing the amino acids in normal TFE3 against those in its mutated counterparts, the researchers found that the mutated forms were more adept at binding to an enzyme critical for transcription, RNA polymerase II. This connection suggests a direct pathway through which these mutations promote tumor activity.
A Broader Implication for Cancer Research
As curiosity piqued the researchers, they examined a database of known oncofusion mutations across various cancers. To their surprise, they found similar amino acid patterns in other oncofusions, indicating that this mechanism may not only pertain to tRCC but could also influence many forms of cancer. Disrupting these interactions presents a tantalizing avenue for future cancer treatments, a path that the Sabari Lab intends to explore further.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)