Breakthrough Study on Calcium Channels Resolves Decades-Long Debate and Unveils Surprising Insights
2024-12-23
Author: John Tan
Summary
In a groundbreaking study published in the Proceedings of the National Academy of Sciences, researchers have finally addressed a long-standing controversy surrounding the molecular mechanisms involved in calcium signaling—essential for a plethora of physiological responses within the human body. This revelation marks a significant milestone in our understanding of how cells regulate crucial processes, including gene expression, apoptosis (programmed cell death), and various immune responses.
Calcium Signaling and SOCE
Calcium signaling acts as a pivotal pathway that enables cells to communicate and respond to their environment. One of the key processes within this signaling realm is store-operated calcium entry (SOCE), which has remained shrouded in mystery, particularly regarding the role of transient receptor potential canonical (TRPC) channels in this mechanism.
Research Overview
Dr. Murali Prakriya, the Magerstadt Professor of Pharmacology and co-corresponding author of the study, stated, "This study addresses a historical controversy surrounding store-operated calcium entry, a fundamental process present in all cell types and crucial to the immune system." Previous research identified a new class of ion channels, known as Orai channels, in mediating SOCE as early as 2006. However, the partnership of Orai channels with TRPC channels in facilitating this essential process remained unclear for years.
Key Findings
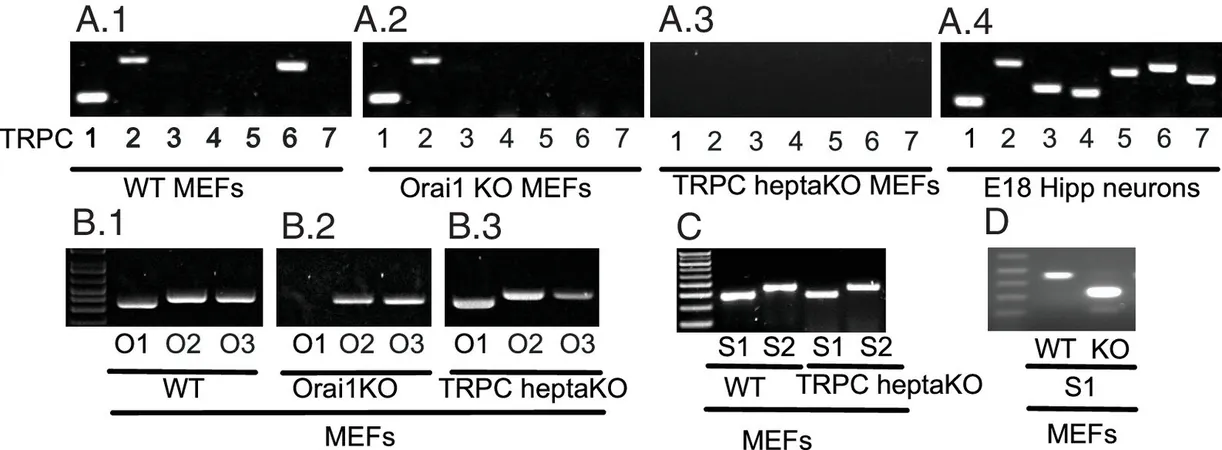
In this revolutionary study, Prakriya and his team utilized genetically engineered mice that lacked all TRPC genes to investigate the role of these channels. The results were astonishing: despite the absence of all seven TRPC genes, the activation of SOCE channels was solely dependent on Orai1 molecules in these mice, suggesting that TRPC proteins are not integral to the calcium replenishment process.
“Employing advanced electrophysiological techniques, we demonstrated that the elimination of all seven mouse TRPC channels does not impact store-operated or receptor-operated calcium entry,” Prakriya noted. This finding effectively resolves a debate that has lingered among researchers for decades, eliminating the assumption that TRPC proteins were essential for cellular calcium signaling.
Implications of the Study
Equally surprising is the discovery that these genetically modified mice not only survived without TRPC channels but also displayed distinct phenotypes. Notably, they exhibited signs of obesity, suggesting that TRPC channels may play a significant role in regulating cellular and body metabolism, opening up new avenues for future research.
These findings not only reshape our understanding of calcium signaling but also herald potential therapeutic implications for a range of calcium-related disorders. As researchers continue to unravel these intricate biological mechanisms, the quest for innovative treatment strategies could be on the horizon, promising to advance medical science significantly.
Conclusion
As the scientific community absorbs the implications of this research, one thing is clear: our grasp of cellular mechanisms is becoming increasingly refined, and the mysteries of cell signaling are poised for further exploration.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)