Breakthrough Study Links Cancer Pathway to Critical Eye Barrier
2025-07-16
Author: John Tan
In a groundbreaking study from the University of Minnesota Medical School, researchers have uncovered a surprising connection between the p53 cancer pathway and the blood-retina barrier, shedding new light on potential cancer treatments.
The p53 gene is pivotal in regulating cell division and suppressing tumors. However, mutations in this gene, which occur in roughly 50% of cancers, can lead to unchecked cell growth and facilitate cancer development. Originally identified in the 1970s as an oncogene, p53 was later reclassified as a tumor suppressor, igniting over four decades of research aimed at harnessing its power against cancer.
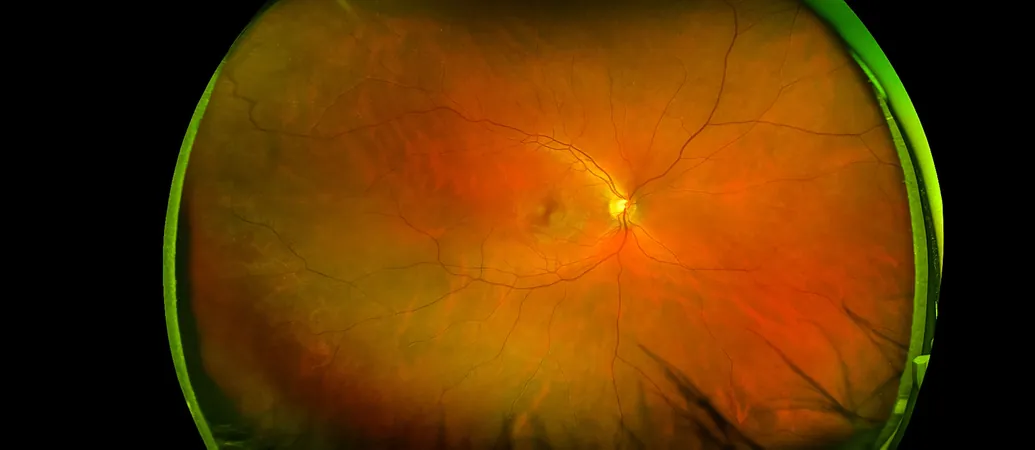
The study published in Science Signaling zeroed in on gene expression within endothelial cells in mice, focusing on how p53 interacts with MDM2 inhibitors and the Norrin/Frizzled4 signaling pathway—crucial for maintaining the integrity of blood-brain and blood-retina barriers.
MDM2 inhibitors are currently being explored as a therapeutic strategy to enhance p53's tumor-suppressing capabilities. These inhibitors aim to disrupt the relationship between MDM2 protein and p53, particularly in advanced solid tumors and certain blood cancers.
However, this new study revealed that MDM2 inhibitors inadvertently compromise the Norrin/Frizzled4 signaling pathway by reducing the levels of a protein called NCAPH, which is essential for DNA organization during cell division. This disruption has significant implications, as the Norrin/Frizzled4 pathway is vital for forming normal vascular structures and sustaining blood-brain and blood-retina barrier integrity.
Researchers also discovered that reducing MDM2 in adult mice led to leaky retinal blood vessels and increased inflammation, raising concerns that MDM2 inhibitors may lead to ocular edema, an unwanted side effect.
According to Dr. Harald Junge, one of the study's authors, "Our findings reveal an unexpected link between the p53 stress response pathway and Norrin signaling in the central nervous system's vasculature. This could have profound implications for cancer therapies targeting MDM2 that may unintentionally disrupt barrier functions, leading to neuroinflammation and transport issues between blood and the central nervous system."
Additionally, the study posits NCAPH as a potential key player linked to familial exudative vitreoretinopathy (FEVR), a rare genetic eye disorder affecting retinal blood vessel development. The researchers advocate for further exploration of NCAPH's role in endothelial cells as both a downstream mediator of p53 and a critical gene in vascular diseases like FEVR.
This pivotal research received funding from the National Eye Institute and the National Institutes of Health, marking a significant step forward in understanding the complexities of cancer treatment and its unintended consequences.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)