Breakthrough Silver-Silica Catalyst Revolutionizes CO2 Conversion and pH Control!
2024-11-15
Author: Li
Introduction
A groundbreaking study led by Dr. Hyung-Suk Oh and Dr. Woong Hee Lee at the Clean Energy Research Center of the Korea Institute of Science and Technology (KIST) has unveiled a remarkable silver-silica composite catalyst. This innovative catalyst offers reversible local pH control, transforming the landscape of CO2 conversion technology and drawing inspiration from Earth's natural geochemical processes.
Background and Significance
Published in the prestigious journal *Energy & Environmental Science*, this research reflects an intriguing parallel to the carbonate-silicate cycle, which plays a crucial role in Earth's carbon regulation. In this cycle, carbon dioxide (CO2) is absorbed from the atmosphere into weathered minerals, only to be released again through volcanic activity. As silicate rocks weather, they generate dissolved silica (SiO2), leading to the formation of carbonate rocks—a process that ultimately contributes to regulating Earth's climate.
Advancements in CCU Technology
In the realm of Carbon Capture and Utilization (CCU) technology, silver catalysts have emerged as highly efficient means for converting CO2 into carbon monoxide (CO), a critical feedstock for various petrochemical products. However, their widespread commercial use has been hampered by challenges such as particle agglomeration at high current densities, which significantly decreases their efficiency and selectivity.
Innovative Catalyst Development
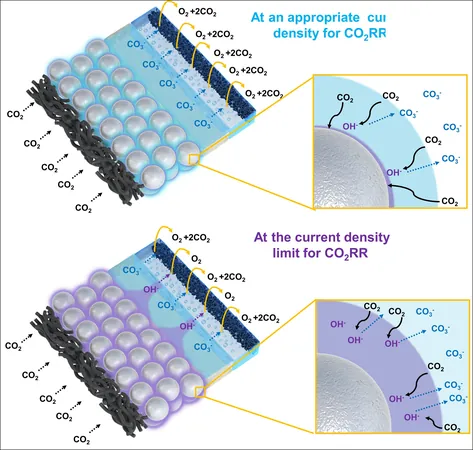
To tackle these hurdles, the research team created a novel silver-silica composite catalyst. This catalyst employs a unique silica-hydroxide cycle, where hydroxide ions (OH-) produced during reactions interact with silica, promoting the dissolution into silicate form and subsequent precipitation under neutral conditions. This method effectively manages pH levels without compromising the catalyst's integral structure.
Performance Achievements
Remarkably, this new catalyst maintained nearly 100% selectivity even under high current densities of 1 A cm-2—an impressive achievement compared to conventional silver catalysts that see their selectivity plummet to approximately 60% at 800 mA cm-2. Furthermore, it demonstrated a substantial enhancement in CO2 conversion to CO, achieving a 47% increase in efficiency, even when tested at elevated current densities.
Future Implications
The implications of this development are profound, signaling a major leap forward for the commercial viability of CCU technology. With its remarkable durability and selectivity stemming from reversible cycling, this catalyst could significantly boost economic feasibility and productivity in electrochemical CO2 conversion processes.
Next Steps
Looking ahead, the research team intends to refine the production methods for these high-performance catalysts and engage in long-term durability testing. Potential applications for this technology are tantalizing, with industrial sectors such as power plants and petrochemical facilities standing to benefit greatly.
Conclusion
Dr. Oh emphasized the importance of this research, stating, "Our findings signify a pivotal moment for enhancing catalyst reversibility and environmental control in electrochemical systems. We anticipate this will play a crucial role in the future demonstration and commercialization of these technologies." As the world grapples with the pressing issue of climate change, innovations like this silver-silica composite catalyst could serve as game-changers in the quest for sustainable energy solutions.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)