Breakthrough: Researchers Convert Methane to Acetic Acid with Ease!
2025-06-10
Author: Arjun
Revolutionary Methane Transformation Under Mild Conditions!
Scientists have just unlocked an exciting new method for converting methane (CH4) into acetic acid (CH3COOH), a high-value chemical, using mild conditions! This groundbreaking research presents a game-changing solution for upgrading natural gas into liquid chemicals that are easy to transport.
The challenge of converting methane has long been the difficulty of breaking its strong C-H bonds, alongside the complexities of activating oxygen and achieving effective C-C coupling reactions. But a team led by Prof. Deng Dehui and his colleagues from the Dalian Institute of Chemical Physics has risen to this challenge.
A Breakthrough Catalyst!
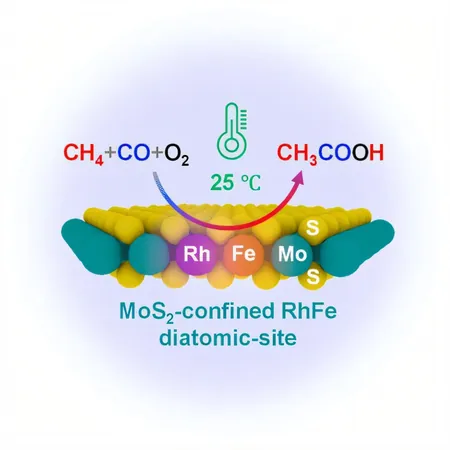
Their study, published in the prestigious Journal of the American Chemical Society, highlights a phenomenal innovation: the use of a MoS2-confined Rh-Fe dual-site catalyst. This innovative catalyst reached an astonishing selectivity of 90.3% for acetic acid, with a productivity rate of 26.2 µmol gcat⁻¹ h⁻¹, all at a comfortable room temperature of just 25°C!
How Does It Work?
The secret lies in the unique structure of the catalyst. The confined iron (Fe) sites play a crucial role in activating oxygen to create highly reactive Fe=O species. This key reaction allows them to dissociate methane into CH3 species, which then pair up with carbon monoxide (CO) on neighboring rhodium (Rh) sites to produce the crucial CH3CO intermediate, leading to the desired acetic acid.
A New Era of Catalyst Design!
Prof. Deng states, "Our study opens up new avenues for designing efficient catalysts for the oxidative carbonylation of methane to acetic acid." This breakthrough not only paves the way for innovative chemical processes but also holds promise for a more sustainable future in energy and chemical production.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)