Breakthrough Method Reveals Heart Dynamics: A Game-Changer for Arrhythmia Research!
2025-06-10
Author: Ming
Revolutionizing Heart Cell Research
In an exciting leap for cardiovascular research, scientists have unveiled an innovative technique that allows them to simultaneously observe electrical and calcium signals in individual heart muscle cells derived from patient-specific induced pluripotent stem cells (iPSCs). This pioneering method could reshape our understanding of heart conditions and how to tackle them.
The Team Behind the Discovery
This groundbreaking study, led by Associate Professor Yoshinori Yoshida from the Department of Cell Growth and Differentiation at Kyoto University, in collaboration with Takeda Pharmaceutical Company, was published in the journal Frontiers in Physiology. Their work sheds light on the complex dynamics of the heart that have puzzled researchers for years.
Understanding CPVT: A Life-Threatening Condition
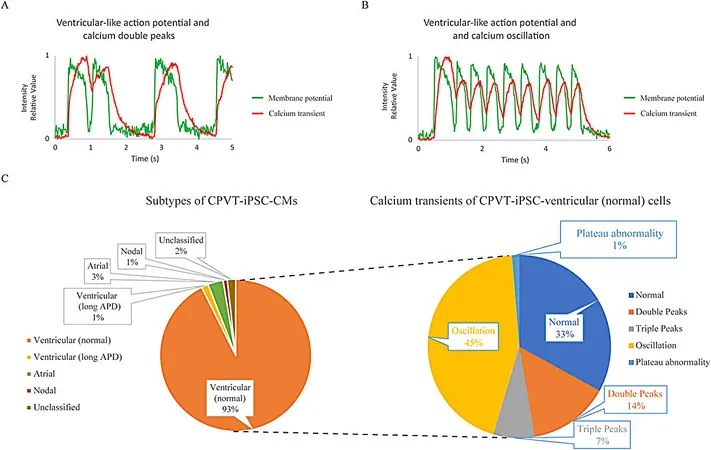
Catecholaminergic polymorphic ventricular tachycardia (CPVT) poses a rare yet severe threat to those with this inherited arrhythmia, characterized by erratic calcium handling in cardiomyocytes. While existing models using human iPSC-derived heart cells have helped in understanding CPVT, traditional studies have often isolated either electrical action potentials or calcium signals—creating a gap in our comprehension of their interrelated effects.
A Cutting-Edge Dual-Dye Approach
To bridge this gap, the research team utilized a clever combination of the FluoVolt membrane potential dye and the newly developed calcium indicator Calbryte 590 AM. This innovative dual-dye approach significantly elevates the signal quality compared to standard practices, enabling prolonged observation of both action potentials and calcium transients in single cells without causing damage. The researchers meticulously adjusted light intensity and filter settings to obtain the best possible results.
Uncovering Abnormalities in CPVT Cells
By applying their method to cardiomyocytes derived from a patient with CPVT type 1 (RyR2-I4587V), the researchers observed troubling abnormalities. Approximately 66% of the tested cardiomyocytes displayed disturbing calcium patterns, such as unexpected double or triple peaks, far exceeding those seen in healthy cells—a clear signature of CPVT.
Drug Testing: Promising Results!
Following these findings, the team explored the impacts of various medications on the defective heart cells. Carvedilol, a widely used nonselective β-blocker, showed some promise at low doses, correcting calcium abnormalities, although higher doses led to undesirable side effects. Flecainide, an antiarrhythmic medication, showed moderate success, echoing real-world clinical observations.
The RyR2 modulator JTV519 (K201) made a significant impact on calcium irregularities at 3 µM, even though it altered action potential shapes. Remarkably, the CaMKII inhibitor KN-93 stood out, effectively normalizing calcium dynamics in 93% of cases at a mere 1 µM, without altering action potentials—an impressive feat!
A New Era in Cardiovascular Treatment?
This new method represents a monumental step forward, enabling researchers to better understand the complex interplay between electrical activities and calcium signaling in heart cells. By providing more accurate modeling of drug responses, it holds the potential to pave the way for safer and more effective treatments tailored to individual patient needs, specifically for challenging arrhythmias.
Looking to the Future
As one of the first methods delivering stable, long-term dual imaging of single human iPSC-derived cardiomyocytes, this breakthrough could extend beyond CPVT. It may open doors to new explorations in various heart disorders and drug-induced arrhythmias, laying the groundwork for future advancements in precision cardiology. The future of heart health research is bright!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)