Breakthrough in Ultrafast Science: New Light Source Transforms Our Understanding of UV-Induced Molecular Reactions!
2024-10-25
Author: Ming
Introduction
In a groundbreaking development, the Attosecond Science group at the Center for Free-Electron Laser Science has engineered a revolutionary light source that emits incredibly short pulses, allowing scientists to investigate UV-induced molecular dynamics with unparalleled temporal resolution. Their remarkable findings have been documented in a recent article published in *Nature Communications*.
Importance of UV Radiation
Ultraviolet (UV) radiation, a common phenomenon, plays a significant role in triggering essential photochemical and photobiological processes, including critical DNA damage. Historically, the inability to generate extremely brief UV pulses has limited our comprehension of the crucial ultrafast mechanisms that unfold during the initial interactions between light and molecules.
The Magic of Femtoseconds
It is in the first few femtoseconds following light absorption where the real magic happens; this is when electrons and nuclei engage in simultaneous motion, setting the stage for molecular reactivity. To unlock new reaction pathways, scientists need to fully understand the UV-induced processes that occur in this minuscule time frame.
Research Objectives
The research team sought to determine if they could manipulate these ultra-fast reactions to influence molecular behavior over much longer timescales. They specifically aimed to understand whether UV excitation occurs swiftly enough to take place before any significant movement of the molecular nuclei.
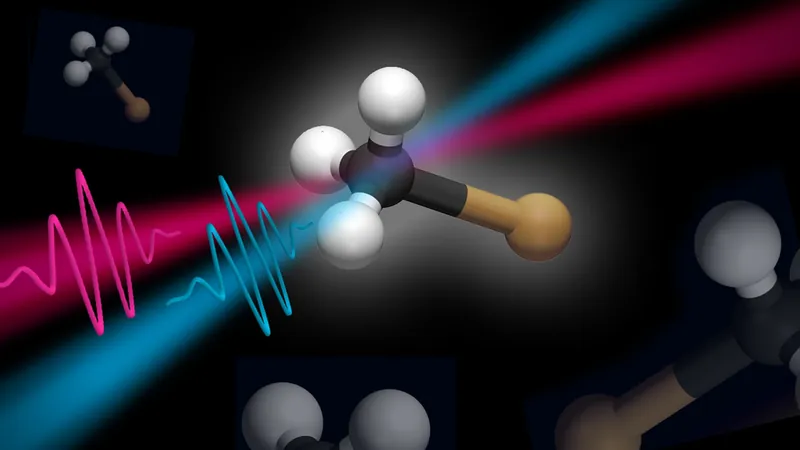
Innovative Experimentation
By employing their innovative light source to conduct experiments on iodomethane—a commonly used benchmark molecule in ultraviolet spectroscopy—they made a significant discovery. Within a critical window of approximately 5 femtoseconds after the molecule is excited, the application of a second laser pulse can successfully inhibit fragmentation.
Insights from the Research Team
"This phenomenon had never been observed before, primarily due to the extended duration of UV pulses that are typically utilized in experimental laser settings," explains Francesca Calegari, head of the Attosecond Science group and professor at the University of Hamburg. Without this second pulse, the molecule would inevitably disintegrate.
Co-author Vincent Wanie, a scientist in the Attosecond Science group, adds, "Our experimental findings have validated a theoretical model that provides invaluable insight into the UV-induced molecular response. This includes determining the time needed to reach the critical C–I bond internuclear distance, where the two electronic states of the neutral molecule can exchange populations."
Collaborative Transformation of Photochemistry
The collaborative effort between experimental and theoretical approaches illustrates how these femtosecond UV pulses could radically transform the landscape of photochemistry. The scientists have unveiled a groundbreaking photoprotection strategy that could prevent molecular dissociation by intervening in the crucial moments following photoexcitement.
Future Implications
This pioneering work not only highlights the capabilities of advanced laser technology but also opens the door to a deeper understanding of ultrafast processes, which could lead to innovative developments across various fields, from photochemistry to materials science and beyond. Buckle up, as this scientific leap could potentially reshape the future of molecular manipulation!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)