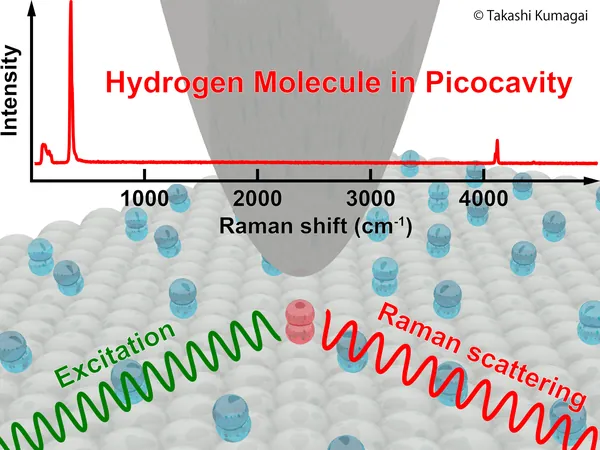
Breakthrough in Quantum Research: Single-Molecule Spectroscopy Unveils Isotope Effects of Hydrogen in Picocavities
2025-05-21
Author: Ming
A Revolutionary Observation in Quantum Science
In a pioneering effort, an international research team has achieved an extraordinary feat—single-molecule spectroscopic examination of hydrogen (H2) and deuterium (D2) confined within an ultra-small picocavity. This innovation was made possible by utilizing tip-enhanced Raman spectroscopy (TERS) between a silver nanotip and a silver single-crystal substrate, all under the most extreme cryogenic conditions.
The Scientific Team Behind the Breakthrough
The research was orchestrated by a collaboration of experts, including Akitoshi Shiotari from the Fritz Haber Institute of the Max Planck Society (Germany), Mariana Rossi from the Max Planck Institute for the Structure and Dynamics of Matter (Germany), and Takashi Kumagai from the Institute for Molecular Science/SOKENDAI (Japan). Their findings have been highlighted in the prestigious journal Physical Review Letters.
Unlocking the Mysteries of Picocavity Interactions
The booming field of nanoscience is increasingly captivated by light-matter interactions within atomic-scale volumes, or picocavities. These tiny electromagnetic field zones, created via plasmon resonance, are seeing rapid advancements, particularly in atomic-scale measurements and quantum photonic technologies.
Unveiling Vibrational Secrets of Hydrogen
This study zeroes in on hydrogen, the most fundamental molecule, confined within a picocavity. High-resolution TERS allowed researchers to explore its vibrational and rotational modes with unmatched precision. Astonishingly, they discovered that the extreme spatial confinement within the picocavity significantly alters the structural and vibrational characteristics of a single hydrogen molecule.
A Surprising Isotope Effect
By fine-tuning the gap between the silver tip and substrate, researchers uncovered an isotope-dependent effect: only the vibrational mode of H2 showcased significant alterations, while D2 remained unchanged. This striking revelation couldn't be detected through traditional ensemble-averaged Raman spectroscopy.
The Science Behind the Discovery
To delve into the underlying mechanisms of this isotope effect, the team employed theoretical simulations, including density functional theory (DFT) and path-integral molecular dynamics (PIMD). Their calculations indicated that the spectroscopy's sensitivity is profoundly influenced by the local interaction potentials surrounding the molecules, particularly driven by van der Waals forces.
Quantum Implications of the Findings
Low-temperature quantum delocalization—the fascinating quantum swelling effect—was vital in explaining the notable differences in vibrational spectra between H2 and D2, highlighting distinct equilibrium positions within the picocavity.
Expert Insights on the Groundbreaking Research
Dr. Rossi expressed amazement at how vibrational coupling and quantum effects intertwined to produce such a pronounced isotope effect. Dr. Shiotari remarked that this advancement deepens our comprehension of light-molecule interactions and enriches our understanding of quantum dynamics in tightly confined environments. Prof. Kumagai also emphasized the future potential of these findings for groundbreaking applications in hydrogen storage, catalytic processes, and next-generation quantum technologies.
The Future of Nanoscale Sensing and Quantum Technologies
As this research paves the way for advanced molecular analysis and quantum control at the individual molecule level, it promises to significantly enhance the fields of nanoscale sensing and quantum photonic technologies.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)