Breakthrough in Photochemistry: Manganese Challenges Precious Metals
2025-09-09
Author: Mei
Game-Changer in Photocatalysis!
For years, precious metals like ruthenium and iridium have dominated the photocatalysis field, thanks to their ability to absorb visible light and retain that energy long enough to facilitate significant chemical reactions. However, their rarity and high costs have held back broader applications such as solar fuel production, creating a pressing need for alternatives.
Manganese to the Rescue!
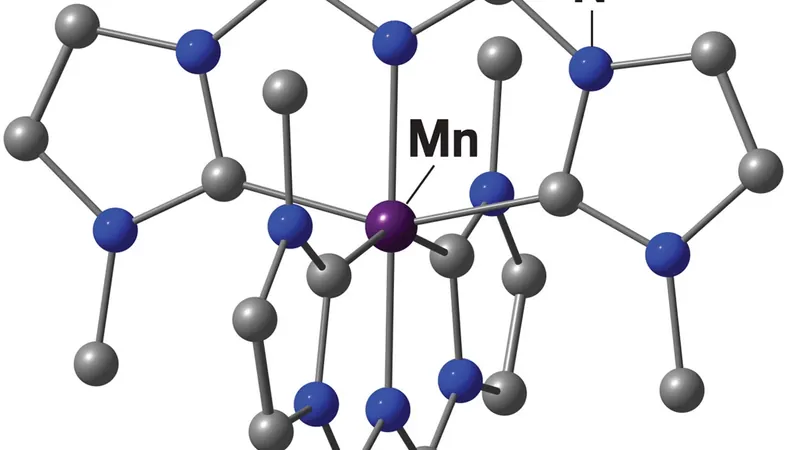
Enter a revolutionary discovery from researchers at Johannes Gutenberg University Mainz: a manganese complex that retains energy for an astonishing 190 nanoseconds after light absorption. This remarkable duration falls within the realms usually associated with noble metals, marking a historic achievement for low-cost materials in practical light-driven chemistry.
Katja Heinze, the lead researcher, attributes the success to the unique combination of manganese with a specially designed carbene-pyridine ligand. "Manganese binds this ligand more effectively than other abundant metals, significantly delaying energy loss," Heinze explains.
Innovation from History
Interestingly, this ligand isn't new; it was created over two decades ago to delve into fundamental coordination chemistry. Initially used with iron-based absorbers by Kenneth Wärnmark's group, this ligand's revival for manganese plays a crucial role in the breakthrough. Wärnmark notes that using second or third-generation ligands could have compromised the long-lived excited state.
Practical Applications Await
Heinze highlights the practicality of this ligand: it's commercially available, making it feasible for large-scale applications. The shift from iron to manganese has extended the lifespan of the excited state from a mere trillionth of a second to 190 billionths—enough time for meaningful interactions with other molecules.
Durability Like Never Before
What's more, this manganese complex showcases about 100 times greater resistance to light-induced degradation compared to traditional benchmarks like ruthenium bipyridine complexes. This durability signals a bright future for persistent light-driven processes.
A Path Forward
However, the journey isn't over. Both Heinze and Wärnmark stress that significant work still lies ahead. "The next step is to modify the system for regeneration without losing that groundbreaking energized state," says Heinze.
With the global shift away from fossil fuels, the need for scalable solar fuel production has never been more urgent. Wärnmark concludes, "Noble metals simply can’t meet this demand, and this innovative manganese complex holds potential for revolutionizing the field of photocatalysis." But, he cautions, there's still a long road before this complex can function in a continuous catalytic cycle.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)