Breakthrough in Hypothyroidism Treatment: Could Cell-Based Therapy be the Future?
2024-11-07
Author: Yu
Breakthrough in Hypothyroidism Treatment: Could Cell-Based Therapy be the Future?
In an exciting development for the treatment of hypothyroidism, teams of researchers from the Center for Regenerative Medicine (CReM) and Boston University (BU), alongside Boston Medical Center (BMC), have unveiled a promising new approach. This innovative research aims to create effective therapies for hypothyroidism, a condition where the thyroid gland fails to produce sufficient hormones, leading to potentially debilitating symptoms.
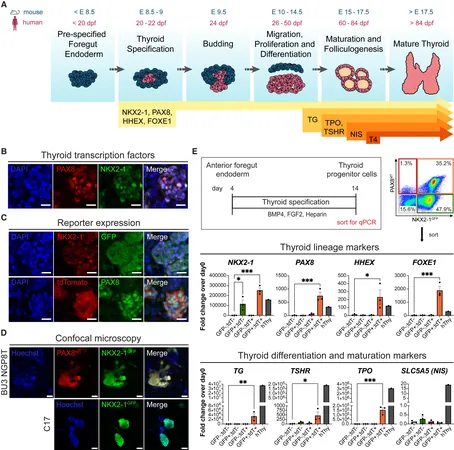
Led by Dr. Darrell Kotton and Dr. Anthony Hollenberg, the groundbreaking study highlights a successful method for generating mature thyroid follicular epithelial cells (TFCs), the key players in thyroid hormone production. Their findings, recently published in Stem Cell Reports, suggest that these lab-produced TFCs have the potential to be transplanted into animal models that mimic the thyroid-deficient state, essentially laying the groundwork for future human applications.
Hypothyroidism can lead to a series of harsh symptoms, including fatigue, muscle weakness, depression, cognitive decline, and a slowed heart rate. By successful transplantation of these TFCs into affected models, the research team is optimistic that human patients could one day find significant relief from their symptoms.
Central to this study is the use of human induced pluripotent stem cells (iPSCs). These remarkable cells are derived from adult skin or blood samples and reprogrammed to behave like embryonic stem cells, with the capacity to transform into any cell type. Dr. Kotton, founding director of CReM, emphasized the importance of iPSCs in regenerative medicine, stating, "They provide an unlimited source of patient-specific cells that can potentially treat various diseases."
Through their meticulous work, researchers managed to create human iPSCs that glow under specific light when they possess thyroid-specific genes, NKX2-1 and PAX8. This allowed the team to accurately identify and isolate TFCs as they developed. By employing a carefully crafted differentiation process, utilizing serum-free media and crucial signaling molecules like BMP4 and FGF2, the team witnessed a remarkable success rate. Within just one month, single-cell RNA sequencing indicated that most iPSCs had effectively turned into mature TFCs, even organizing themselves into functional three-dimensional thyroid follicles.
The ultimate test came when the new TFCs were transplanted into thyroid-deficient animals. Encouragingly, these cells developed into thyroid-like structures, producing the necessary thyroglobulin-positive follicles characteristic of healthy thyroid function.
Dr. Hollenberg underscored the significance of these findings, noting that while the journey to clinical application requires further research, the progress made thus far signifies a monumental step towards potential regenerative treatments for hypothyroidism. "One day, we aspire to provide a cell-based solution for patients who have undergone thyroid surgery or who are affected by congenital hypothyroidism," he added.
As researchers continue to refine their methods, the hope for patients suffering from hypothyroidism grows brighter. The possibility of utilizing iPSC-derived cells in clinical settings is not just a medical breakthrough—it represents a frontier of innovation in the pursuit of effective treatments for chronic health issues. Stay tuned as we follow this promising development that could change lives!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)