Breakthrough in Hydrogen Energy: Chainmail Integrated-Electrode Shows Promise for Efficient Hydrogen Sulfide Electrolysis!
2025-03-20
Author: Ming
Introduction
In a significant stride towards sustainable energy solutions, researchers have developed an innovative chainmail integrated-electrode designed for the efficient electrolysis of hydrogen sulfide (H2S). This toxic byproduct of fossil fuel extraction not only poses severe environmental challenges but also represents an untapped resource for green energy production.
Historical Context
Historically, the conventional Claus process converts H2S into elemental sulfur but overlooks the potential of recovering hydrogen gas. This oversight signifies a missed opportunity for generating eco-friendly energy. Now, electrocatalytic decomposition of H2S offers a dual benefit: the elimination of harmful pollutants while simultaneously producing green hydrogen, a key player in renewable energy strategies.
Research Development
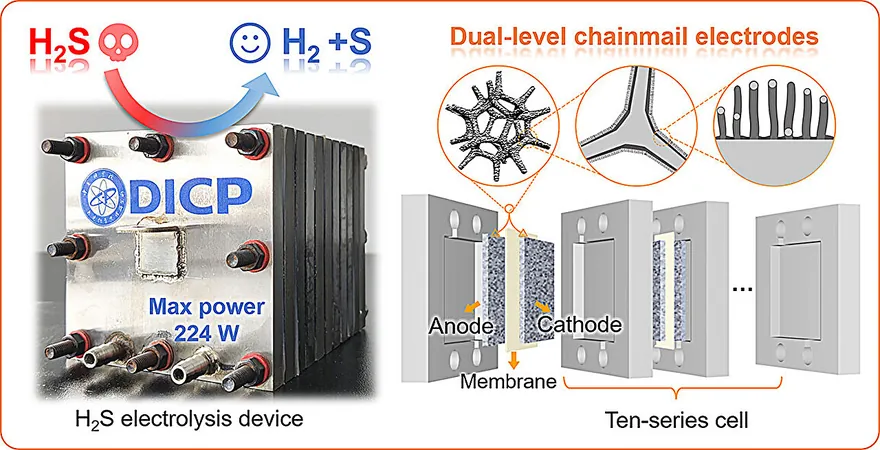
A recent study published in *Angewandte Chemie International Edition* highlights a novel development from a research team led by Prof. Deng Dehui and Assoc. Prof. Cui Xiaoju at the Dalian Institute of Chemical Physics, part of the Chinese Academy of Sciences. They engineered a groundbreaking dual-level chainmail integrated-electrode that excels in hydrogen production through H2S electrolysis.
Innovative Design
The researchers employed a cutting-edge design of graphene-encapsulated nickel foam (Ni@NC foam), featuring a highly effective chainmail structure that significantly enhances both catalytic activity and durability. Notably, this electrode achieved an industrial-scale current density exceeding 1 A/cm² at a low voltage of 1.12 V, a performance five times better than traditional nickel foam electrodes. Remarkably, the Ni@NC foam maintained stability for over 300 hours, boasting a lifespan at least ten times longer than its commercial counterparts.
Real-World Applications
In real-world applications, particularly in natural gas desulfurization tests, the integrated-electrode effectively oxidized and removed 20% of H2S at the anode while producing sulfur powder simultaneously. This process not only cleans up the toxic byproduct but also collects high-purity hydrogen at the cathode. Compared to conventional water electrolysis methods, this innovative system slashes energy consumption by an impressive 43% at a current density of 200 mA/cm², paving the way for a more sustainable approach to hydrogen production.
Conclusion and Implications
Prof. Deng expressed excitement regarding the study’s implications: "We are offering an efficient, low-energy solution for natural gas purification and demonstrating the potential of converting H2S into valuable hydrogen fuel for industrial applications." This breakthrough has the potential to reshape hydrogen production technologies and accelerate the transition towards cleaner energy solutions in industries reliant on fossil fuels!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)