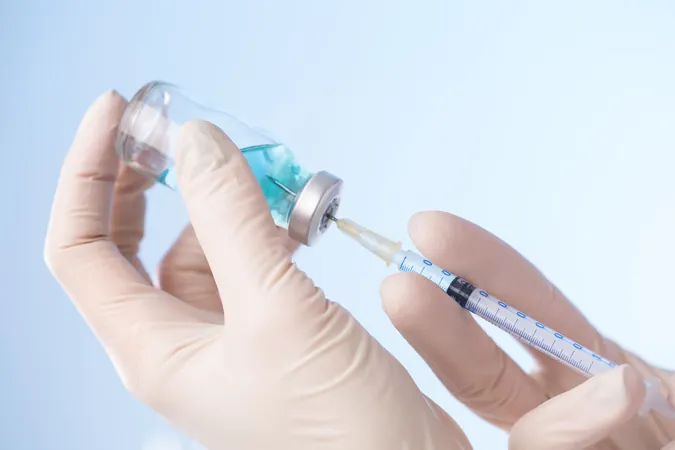
Breakthrough in Hepatitis E Vaccination: Two Doses Show Remarkable Efficacy in Sudan Outbreak
2025-01-22
Author: Mei
Breakthrough in Hepatitis E Vaccination
In a groundbreaking study published in The Lancet Infectious Diseases, researchers have discovered that a two-dose regimen of the Hecolin vaccine (HEV 239; developed by Xiamen Innovax Biotech Co Ltd) is highly effective against the hepatitis E virus (HEV) genotype 1 amid an ongoing outbreak in Sudan. This finding comes as a significant relief; the simplified vaccination schedule promises greater accessibility compared to the traditional three-dose regimen.
Understanding Hepatitis E's Impact
Hepatitis E, primarily transmitted through contaminated food and water, is a leading cause of acute viral hepatitis and has led to substantial outbreaks across Asia and Africa. Although estimating the global burden of hepatitis E is complicated due to inadequate clinical surveillance, researchers have suggested over 70,000 deaths were attributed to HEV genotypes 1 and 2 in 2005 alone, with current estimates indicating as many as 50,000 deaths annually.
Importance of Effective Vaccination
"Symptoms of hepatitis E often mimic those of other acute jaundice-causing diseases, complicating diagnosis," said Andrew Azman, an epidemiologist from the Centre for Emerging Viral Disease at the Université de Genève Hôpitaux Universitaires de Genève (UNIGE-HUG). This emphasizes the critical need for effective vaccination and surveillance strategies.
The Two-Dose Regimen Study
Currently, the Hecolin vaccine requires three doses, given at months 0, 1, and 6, which have shown 100% protective efficacy against clinically apparent HEV infections, notably genotype 4. However, this new study assessed a shorter, two-dose schedule aimed at reducing barriers to vaccination.
Study Details and Results
Conducted between May 10 and December 10, 2022, at a Médecins Sans Frontières hospital, the case-control study involved 859 suspected hepatitis E patients, of which 201 met eligibility criteria, including ages 16 to 40 and residing in the Bentiu internally displaced persons camp in South Sudan. Out of those, lab-confirmed cases were noted among individuals who were mostly unvaccinated.
Remarkably, the two-dose vaccination achieved an unadjusted effectiveness rate of approximately 67.8%, which rose to an impressive 84.0% after accounting for potential confounding factors. The analysis also indicated that existing health-seeking behavior may skew effectiveness estimations, potentially suggesting that vaccine effectiveness could be even higher.
Expert Insights
Dr. Iza Ciglenecki, operational research coordinator at Médecins Sans Frontières in Switzerland, remarked, "Our study demonstrates that a two-dose vaccine regimen is particularly effective, showcasing excellent results in challenging conditions, like a displaced persons camp."
Emerging Implications
Furthermore, the study found that under specific conditions, the adjusted effectiveness of the two-dose regimen reached around 89.4%, underscoring its potential role in combating HEV genotype 1 efficiently, especially in emergency scenarios. "These promising results have contributed to the World Health Organization (WHO) approving a stockpile of hepatitis E vaccines for emergency situations, which could save countless lives," added Azman.
Future Outlook
The researchers highlight that the initiative could pave the way for more robust public health responses to hepatitis E, potentially altering the landscape of hepatitis vaccinations as we know it. The authors of the study call for further research to understand the vaccine's effectiveness in diverse populations and various geographic contexts, indicating a hopeful future in the fight against hepatitis E.
Conclusion
In summary, as public health officials and organizations worldwide strive to combat hepatitis E, this study marks a significant step forward in simplifying vaccination strategies that could ultimately reduce the disease's burden on vulnerable populations.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)