Breakthrough in Enzyme Design: Scientists Create Super-Efficient Catalysts
2025-07-14
Author: Nur
Revolutionizing Enzyme Catalysis: A Major Scientific Leap
Researchers at the Weizmann Institute of Science have achieved a groundbreaking milestone in enzyme technology, developing a new computational workflow that produces enzymes with catalytic efficiencies exceeding 100,000 M−1 s−1. This remarkable efficiency rivals that of natural enzymes, all without the need for extensive fine-tuning.
Natural enzymes are renowned for their ability to facilitate biochemical reactions with astonishing swiftness and precision, setting a high bar that artificial catalysts have historically struggled to reach. However, through innovative computational strategies, scientists have created proteins capable of executing reactions beyond natural enzymes' capabilities, though these creations often lag significantly behind their biological counterparts in terms of speed.
The Study: Crafting Efficiency from Scratch
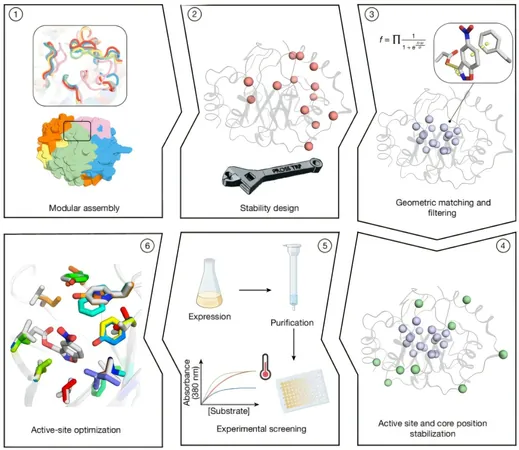
In a pivotal study published in *Nature*, titled "Complete computational design of high-efficiency Kemp elimination enzymes," scientists devised an entirely computational method to engineer stable and effective enzymes with minimal optimization efforts. This approach involved the systematic assembly of thousands of TIM-barrel backbones, one of the most prevalent structures in enzyme proteins, by integrating sequences from similar proteins.
The folding of the central β barrel strategically positions residues toward the active-site cavity, allowing optimal arrangement of catalytic and substrate-binding components.
Innovations in Active-Site Design
The team employed advanced Rosetta atomistic calculations to refine active-site residues, generating millions of enzyme designs. These designs were evaluated based on a multi-faceted objective function balancing energy efficiency and desolvation of the catalytic base. Out of 73 candidates chosen for laboratory trials, 66 were successfully expressed, with 14 demonstrating promising cooperative thermal denaturation behavior.
The introduction of 5-8 mutations in the active sites led to significant enhancements in catalytic performance. Notably, one variant, derived from design 61, achieved a catalytic efficiency of 3,600 M−1 s−1 and a turnover rate of 0.85 reactions per second.
Record-Breaking Efficiency Achieved
Eight of the designs based on a specific template, Des27, exhibited catalytic rates 10 to 70 times higher than their predecessor, with Des27.7 soaring to an astounding 12,700 M−1 s−1 and a kcat of 2.85 s−1. Remarkably, a single-point mutation converting phenylalanine to leucine propelled catalytic efficiency to 123,000 M−1 s−1, with a turnover rate hitting 30 s−1.
Future Implications: A New Era of Biocatalysis
The results underline that advanced atomistic computational methods are now reliable enough to create efficient enzymes with natural folds, eliminating the need for laborious lab screening or artificial intelligence-generated scaffolds. Researchers are optimistic that further progress in theoretical enzyme modeling will pave the way for programmable biocatalysis, unlocking a realm of possibilities in product manufacturing, therapeutic applications, pollution remediation, and green chemistry.
This breakthrough has the potential to significantly alter industrial processes and environmental sustainability, heralding a new era in the field of biocatalysis.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)