Breakthrough in Childhood Cancer Treatment: Avapritinib Targets Aggressive Brain Tumors!
2025-03-27
Author: Daniel
A groundbreaking study has illuminated the potential of avapritinib as a treatment for pediatric high-grade gliomas, especially the aggressive H3K27M diffuse midline gliomas (DMG), known for their grim prognosis. Researchers have identified the role of platelet-derived growth factor receptor alpha (PDGFRA) in the development of these malignant tumors, providing new hope in the fight against these devastating conditions.
Led by Dr. Mariella Filbin, a prominent figure at the Brain Tumor Center of Boston Children’s Hospital and Dana-Farber Cancer Institute, the recent multicentric research unveiled crucial insights linking PDGFRA to the pathogenesis of pediatric high-grade gliomas. The findings, published in the esteemed journal Cancer Cell, offer compelling evidence that PDGFRA not only has genetic alterations prevalent in these tumors but also plays a significant role in tumor growth—making it a potential target for innovative therapies.
Uncovering PDGFRA’s Role
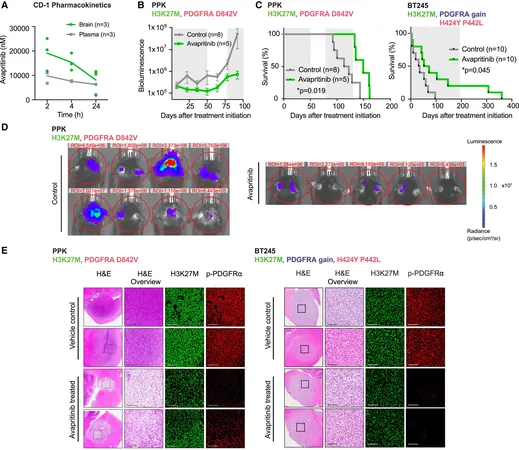
To assess the prevalence of PDGFRA mutations, Dr. Filbin and her team scrutinized genomic data from 217 pediatric high-grade glioma samples, revealing that about 15% of patients harbored PDGFRA alterations. The researchers illustrated that tumors with these mutations exhibited significantly increased PDGFRA expression, solidifying its role in these aggressive cancers.
In a groundbreaking move, the team evaluated the effects of four PDGFRA inhibitors—dasatinib, crenolanib, axitinib, and avapritinib—across glioma cell lines with PDGFRA alterations. Among them, avapritinib stood out for its impressive potency and minimal off-target activity, suggesting fewer side effects, a crucial factor for treating vulnerable pediatric patients.
Early Clinical Success in Treatment
Taking a bold step forward, the researchers expanded their investigation into early-phase clinical applications. They treated eight pediatric and young adult patients with high-grade gliomas under a compassionate use program, primarily focusing on those with DMGs and PDGFRA alterations.
After an average of four months on daily avapritinib treatment, encouraging results emerged: three patients exhibited radiographic responses, and notably, they survived approximately double the duration compared to those without a positive response to the drug, despite the eventual progression of their disease.
This preliminary data not only underscores their safety profile but also opens doors to a hopeful avenue for targeted therapy in pediatric high-grade gliomas, where options are sadly limited.
A New Frontier in Pediatric Oncology
As these findings ripple through the medical community, the spark of potential is igniting further research. The promising results with avapritinib might revolutionize how pediatric high-grade gliomas are understood and treated, bringing a glimmer of hope to families battling this fierce opponent. The quest for more effective, targeted treatments continues, and researchers should eagerly watch how avapritinib performs in larger clinical trials.
In conclusion, this research marks a notable advancement in the treatment of aggressive brain tumors in children. As scientists delve deeper into the intricacies of gliomas, the potential for life-saving therapies becomes increasingly tangible, illuminating a path toward brighter futures for young cancer patients everywhere.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)