Breakthrough in CAR-T Cell Therapy: The Power of 'Memory Cells' Could Revolutionize Cancer Treatment!
2025-01-02
Author: Jia
Introduction
In a groundbreaking study from the University of Colorado Anschutz Medical Campus, researchers have unveiled a fascinating aspect of CAR-T cell therapy that could transform our approach to cancer treatment. They have discovered that certain CAR-T cells, which are engineered to combat cancer, carry a memory of past encounters with pathogens such as bacteria and viruses. This remarkable finding opens the door to creating more tailored and effective cancer therapies.
Study Overview
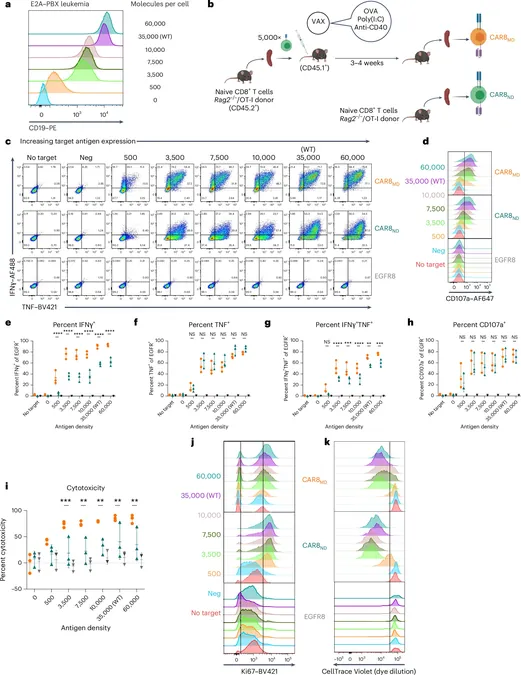
Published in the prestigious journal *Nature Immunology*, the study highlights the effectiveness of chimeric antigen receptor (CAR)-T cells, especially in treating blood cancers like leukemia and lymphoma. The current CAR-T therapy involves extracting T cells—crucial immune warriors—from a patient’s blood, modifying them to target cancer cells, and reinfusing them back into the patient’s system. However, this new research reveals complexities that could enhance this therapy.
Key Findings
The research team, led by the accomplished Dr. Terry Fry, senior author and pediatric oncologist, found that some CAR-T cells retain long-lasting “memories” of their previous interactions with antigens, even after undergoing a rigorous manufacturing process. This means that CAR-T cells with prior antigen exposure respond differently in the body compared to those without any prior contact.
“What was astonishing was how deeply these past interactions are ingrained in the cells,” Fry explained. The study’s lead author, Dr. Kole DeGolier, elaborated on the discovery that different types of CAR-T cells—referred to as "memory cells"—are quicker to attack cancer, but tire out faster, potentially leading to cancer relapse. On the other hand, “naïve” cells, which lack prior antigen exposure, showed promising capabilities for long-term effectiveness and resistance to exhaustion.
Mechanistic Insights
By juxtaposing these two cell types, researchers identified specific genetic targets that could be manipulated to enhance their functionality. Notably, the gene RUNX2 emerged as a critical factor that could be targeted to improve the performance of naïve cells, enhancing their survival and reproductive rate compared to memory cells.
Experimental Results
The initial experiments were conducted on mouse models and then expanded to human cells. Remarkably, T cells taken from vaccinated subjects demonstrated changes after their exposure to vaccine antigens, allowing them to respond more effectively against leukemia. However, once again, they displayed quicker exhaustion than naïve cells—highlighting a crucial balance between immediate attack capabilities and long-term sustainability.
Future Implications
The breakthrough findings hold promise for more precise engineering of CAR-T cells, potentially leading to therapies that not only better target cancer cells but also mitigate the severe side effects often associated with these treatments, such as inflammatory responses that can be debilitating for patients.
“There’s immense potential in understanding these distinctions to develop smarter, more rationally designed therapies,” stated Fry. The study promotes further exploration of RUNX2's role in limiting cell exhaustion, particularly in the challenging context of solid tumors, where fatigue significantly hampers anti-tumor responses.
Conclusion
As CAR-T cell therapies continue to evolve, the implications of these findings could herald a new era in cancer treatment—one where memory and strategy intertwine in the battle against this devastating disease. Stay tuned as researchers work to maximize the potential of these "memory cells" and reshape the future of oncology!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)