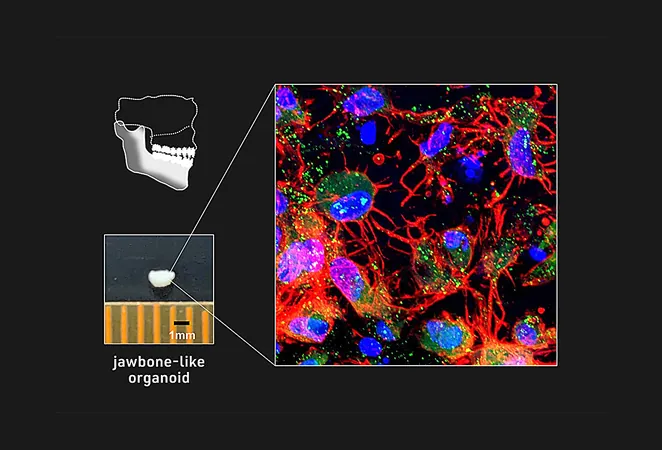
Breakthrough in Bone Health: Jawbone Organoids Created from Patient Cells Could Revolutionize Treatment
2025-07-03
Author: Jia
A Groundbreaking Leap for Bone Disease Research
In an exciting development in biomedical engineering, a team led by Associate Professor Makoto Ikeya has successfully created jawbone-like organoids from induced pluripotent stem (iPS) cells. Their groundbreaking study, published in the prestigious journal *Nature Biomedical Engineering*, could pave the way for innovative therapies for jawbone diseases.
Understanding the Vulnerability of Jawbone
The jawbone, comprising the maxilla and mandible, is the largest biomineralized organ in our mouth and is prone to serious conditions caused by genetic issues, infections, and injuries. Previously, scientists lacked a reliable model to study jawbone development and related diseases—until now.
Transforming Cell Culture Techniques
Ikeya's team had already mastered the art of generating cranial neural crest cells (NCCs) in a 2D cell culture. Building on this foundation, they sought to evolve their method into a three-dimensional (3D) model that accurately represents jawbone tissue.
From Cells to Organoids: The Process
Through meticulous optimization of cell signaling pathways and culture conditions, the researchers successfully induced NCCs to form cell aggregates from various iPS cell lines. This included samples from patients suffering from osteogenesis imperfecta (OI), commonly known as brittle bone disease. By mimicking embryonic development signals, they transformed these aggregates into an intermediate cell type called mandibular prominence ectomesenchyme (mdEM), which closely resembles embryonic tissue.
Building Bone: The Next Steps
The team further nurtured these mdEMs under conditions that promote bone growth, resulting in the creation of mineralized tissue clusters exhibiting all the hallmarks of bone formation. This significant breakthrough implies that these jawbone-like organoids can indeed construct functional bone tissue.
Testing Regenerative Potential in Mice
Taking their research a step further, they transplanted these promising organoids into the bodies of immunocompromised mice, where they developed into highly mineralized, vascularized bone tissues. Although the mineral density wasn’t as robust as traditional grafts, the organoid transplants resulted in notable increases in healthy, vascularized bone tissue.
Modeling Osteogenesis Imperfecta's Effects
Ikeya's team took their research further by generating NCCs from a patient with OI, leading them to explore whether the resulting organoids could effectively model the disease. Within days, they observed the secretion of abnormal collagen and a significant reduction in crucial osteogenic markers in organoids derived from COL1A1 mutant cells—highlighting the pathological characteristics associated with OI.
Hope for Future Treatments
In contrast, organoids from genetically rescued counterparts showed none of the previous issues, suggesting a potential pathway toward treatment. When tested in vivo, the OI-derived organoids resulted in diminished bone formation, demonstrating behavior characteristic of brittle bone disease. This research not only illuminates the complexities of bone diseases but also opens up potential avenues for targeted therapies, moving us closer to tackling these challenging conditions head-on.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)