Breakthrough in ALS Research: A Game-Changer in the Fight Against Neurodegenerative Diseases!
2025-04-17
Author: Arjun
Could This Cellular Repair System Be the Key to Combatting ALS?
Amyotrophic lateral sclerosis (ALS), a rare yet devastating disease, is wreaking havoc on the nervous system. With no cure currently available, ALS relentlessly attacks motor neurons in both the brain and spinal cord, eventually leading to severe muscle paralysis and often leaving patients dependent on wheelchairs. As the condition progresses, simple actions like speaking, swallowing, and even breathing become monumental challenges.
The Culprit: Protein Aggregates and TDP-43
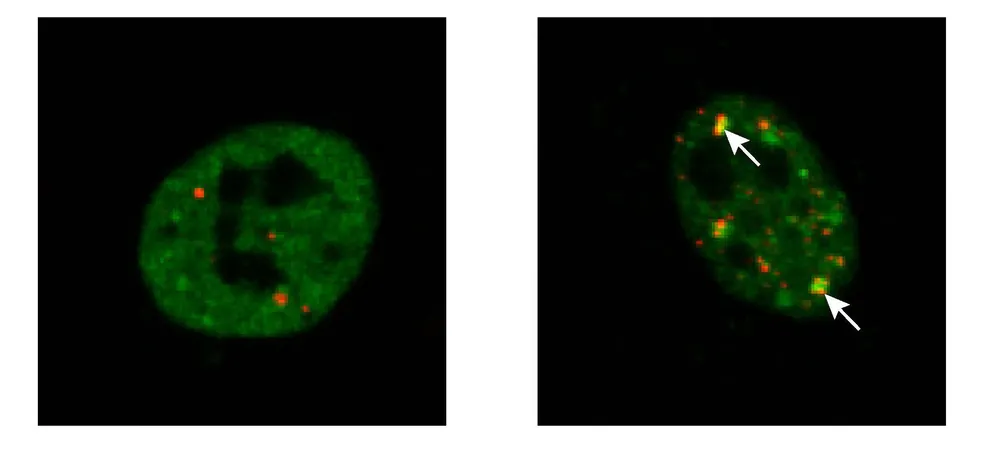
A critical aspect of ALS is the accumulation of harmful protein aggregates in motor neurons. Notably, TDP-43, an essential player in RNA metabolism within healthy cells, transforms into these damaging aggregates in ALS patients. Instead of remaining soluble and functional in the cell nucleus, TDP-43 ends up forming clusters outside the nucleus, leading to the eventual death of motor neurons.
Groundbreaking Research Offers New Hope!
Now, researchers from the Cluster4Future PROXIDRUGS initiative, hailing from the universities of Frankfurt, Mainz, and Kiel, have made a remarkable discovery: a method to thwart the formation of these disastrous TDP-43 aggregates in cultured cells. Scientists Kristina Wagner, Dr. Jan Keiten-Schmitz, and Professor Stefan Müller took innovative approaches, exposing cells to stress conditions, such as elevated temperatures and chemical agents. This triggered the release of TDP-43 from the nucleus, causing it to gather into protective structures known as stress granules.
Understanding Stress Granules: The Double-Edged Sword
Keiten-Schmitz clarifies that while stress granules typically act as a safe haven for proteins during stressful times, a mutation in TDP-43—common in ALS patients—renders these granules detrimental. Instead of serving their protective purpose, these granules become more solidified, ultimately contributing to neuronal damage.
A New Strategy: Keeping TDP-43 in Check!
The groundbreaking study demonstrates a successful method to prevent TDP-43 from leaking out of the nucleus during stress. By hooking TDP-43 to a cellular 'roadside assistance' protein called SUMO, the team effectively directed TDP-43 towards cellular 'mechanics' known as nuclear bodies.
Future Prospects: A New Era in ALS Treatment?
Thanks to this innovative approach, TDP-43 remains soluble while nuclear bodies work on restoring or breaking down the harmful forms through the cellular recycling system. First author Kristina Wagner states, "This mechanism could prevent the formation of insoluble protein aggregates that cause cell damage or death." The research team is now on the hunt for drug candidates that can chemically bond SUMO and TDP-43 together. Müller adds, "Our initial cell culture experiments show great promise. Though there’s a long road ahead toward developing a treatment for ALS, this research path is certainly worth exploring further!"




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)