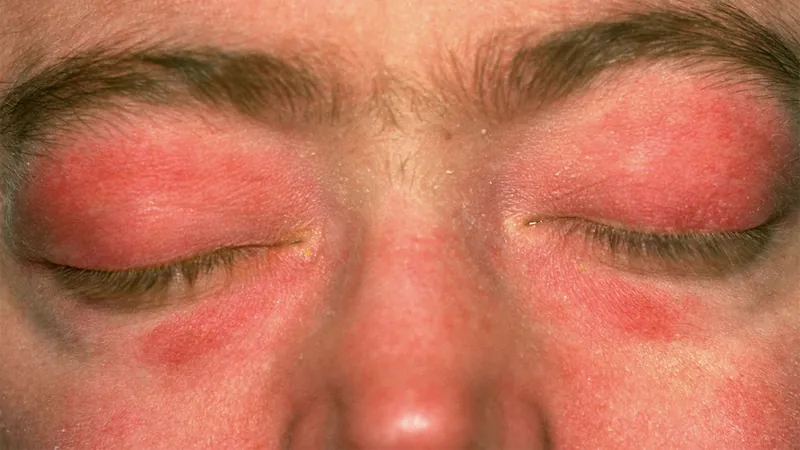
Breakthrough Drug Significantly Reduces Disease Activity in Dermatomyositis Patients
2025-01-15
Author: Sarah
Breakthrough Drug Significantly Reduces Disease Activity in Dermatomyositis Patients
In a remarkable breakthrough for patients suffering from dermatomyositis, an investigational monoclonal antibody, dazukibart, has demonstrated significant efficacy in reducing disease activity. According to recent findings from a randomized phase II clinical trial, this new treatment specifically targeting interferon beta (IFN-β) has yielded rapid and notable benefits for adults afflicted by this chronic autoimmune disorder.
Impressive Results from Clinical Trials
The study, led by Dr. Ruth Ann Vleugels from Brigham and Women's Hospital and Harvard Medical School, revealed that participants with skin-predominant dermatomyositis receiving a dosage of 600 mg of dazukibart experienced a striking improvement, with a decrease of 18.8 points on the Cutaneous Dermatomyositis Disease Area and Severity Index-Activity (CDASI-A) after just 12 weeks. In contrast, the placebo group experienced only a minimal reduction of 3.9 points (P<0.0001).
Furthermore, data from the entire cohort indicated that both doses of dazukibart (600 mg and 150 mg) provided significant improvements in disease activity compared to the placebo: - **600 mg:** -16.3 point difference (P<0.0001) - **150 mg:** -13.7 point difference (P<0.0001) These results mark the first evidence that inhibiting IFN-β can effectively treat dermatomyositis, according to Vleugels and her team, who published their findings in The Lancet.
The Need for Specific Treatments
Dermatomyositis is a difficult condition known for its debilitating symptoms, including skin rashes and muscle weakness, along with systemic issues like interstitial lung disease. Current treatment regimens often involve a combination of immunosuppressants and corticosteroids, but these approaches are frequently inadequate and come with significant side effects. Experts emphasize that the limitations of existing therapies highlight the pressing need for more effective and targeted treatment options that address the unique causes of dermatomyositis.
Dr. Farida Benhadou and Dr. Elie Cogan echoed this sentiment in an accompanying editorial, noting that elevated levels of IFN-β have been closely linked to disease activity in dermatomyositis, marking it as a key target for future treatments.
Positive Safety Profile and Ongoing Research
Dazukibart has shown a promising safety profile, with no major differences in adverse events between treated groups and the placebo. While the drug did not produce significantly different outcomes in the muscle-predominant cohort, numerical improvements were observed across key muscle function metrics.
A total of 125 patients were enrolled in the study, which spanned 25 sites across Europe and the United States. Of those, 75 participants were assigned to receive dazukibart at either 150 mg (15 patients) or 600 mg (37 patients), while 23 received a placebo. Notably, participants were required to have already failed previous systemic treatments to qualify for the study.
As the research progresses, a phase III trial is already underway, targeting patients with active idiopathic inflammatory myopathies, including dermatomyositis and polymyositis, thereby paving the way for broader usage of dazukibart in similar diseases.
Conclusion: A Hopeful Outlook for Patients
The promising results of dazukibart signal a potential new era in the treatment of dermatomyositis, offering hope for many who have battled ineffective therapies in the past. With continued research and clinical trials, dazukibart may soon become a game-changer in the fight against this challenging autoimmune disease, improving both outcomes and quality of life for those affected. Keep an eye on ongoing developments, as this innovative treatment could redefine standard care in the near future!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)