Breakthrough Discovery: Scientists Unravel the Secrets of a Key Human Protease Linked to Major Diseases
2025-06-26
Author: John Tan
Unlocking the Mysteries of Post-Translational Modifications
In the intricate world of cell regulation, post-translational modifications of proteins are critical players. These alterations can significantly change how proteins act, profoundly impacting various physiological processes. Among the most intriguing of these modifications is SUMOylation, where a special protein known as SUMO (Small Ubiquitin-like MOdifier) attaches to other proteins, tweaking their functions and influencing their roles in the body.
The Role of SUMO Proteases
This fascinating biological dance involves numerous proteins, both in activating SUMO and in the modifications that follow. At the forefront of this process are the SUMO proteases, enzymes that not only activate SUMO but also detach it from target proteins when necessary.
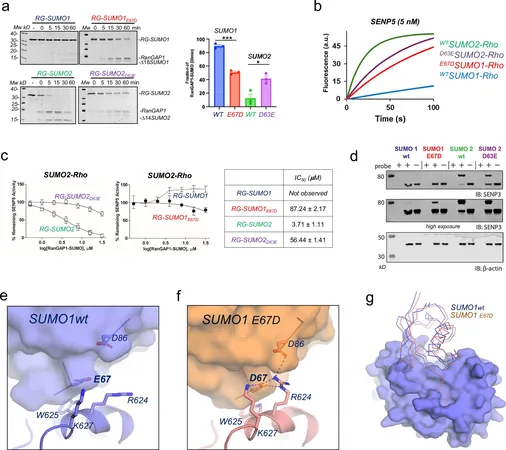
A Game-Changing Study on SENP5 Protease
Recent groundbreaking research from the Institute of Biotechnology and Biomedicine at UAB (IBB-UAB) has shed light on the structural intricacies of SENP5, a human protease linked to a range of diseases. These findings were published in the prestigious journal Nature Communications, paving the way for a deeper understanding of how SENP5 interacts with different forms of SUMO.
Revealing Insights Into SENP5's Structure
The research team unveiled the three-dimensional structure of SENP5 in conjunction with various SUMO types. The analysis revealed a positively charged region of the protease that plays a crucial role in its preference for SUMO2. This structural insight not only highlights key interactions between SENP5 and SUMO2 but also opens new avenues for the development of targeted inhibitors to combat the diseases associated with SENP5 dysfunction.
A New Horizon in Disease Treatment
The implications of this study are vast, offering a promising pathway for future research aimed at designing precise drugs targeting SENP5. As scientists continue to decode the complex mechanisms of proteases like SENP5, the potential for innovative treatments for related diseases grows ever closer to reality.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)