Breakthrough Discovery Reveals RNA-Based Network Influencing Colorectal Cancer and Immune Response
2025-07-08
Author: Nur
Groundbreaking Research Sheds Light on Colorectal Cancer Dynamics
An innovative study led by Professor Gu Hongcang and his colleague Zhang Fan from the Hefei Institutes of Physical Science at the Chinese Academy of Sciences has unveiled a pivotal long non-coding RNA (lncRNA)-driven regulatory network crucial for understanding colorectal cancer (CRC) progression and its interaction with the immune system.
Published in the prestigious journal *Molecular Cancer*, this research highlights promising new therapeutic targets that could potentially overcome the significant treatment resistance faced by CRC patients.
The Challenge of Colorectal Cancer Treatment
Colorectal cancer presents a formidable challenge due to its intricate genetic and epigenetic diversity, complicating the efficacy of current therapies, particularly immunotherapy. While immune checkpoint inhibitors have changed the landscape of cancer treatments, a staggering 85% of CRC patients remain resistant, primarily due to this molecular complexity.
Innovative Multi-Omics Analysis Reveals Key Insights
To unravel the mechanisms behind CRC, the research team conducted a comprehensive multi-omics analysis integrating transcriptomic, proteomic, and metabolomic data from CRC tumors alongside matched normal tissues. Their thorough investigation led to the identification of 1,394 differentially expressed lncRNAs, 2,788 mRNAs, 548 proteins, and 91 metabolites.
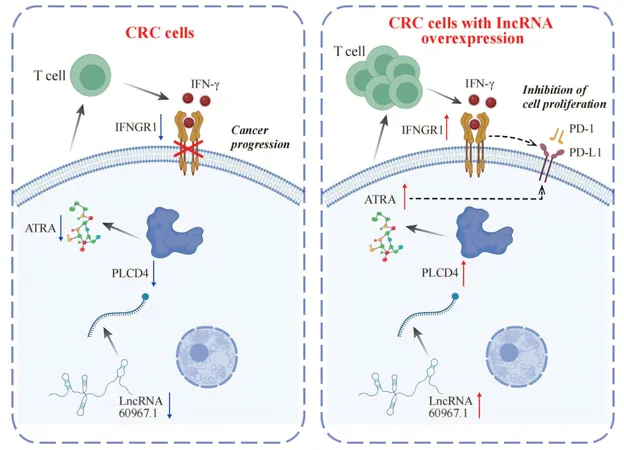
From this extensive dataset, the researchers constructed a regulatory network featuring 22 lncRNAs, 14 mRNAs/proteins, and 9 metabolites. Among these, lncRNA 60967.1 emerged as a standout, demonstrating significant downregulation in both CRC cell lines and patient samples.
Targeting lncRNA 60967.1: A New Approach to Cancer Treatment
Functional experiments revealed that restoring the expression of lncRNA 60967.1 activated the tumor suppressor gene PLCD4 and boosted the levels of all-trans retinoic acid (ATRA). This reactivation intensified apoptosis in cancer cells induced by interferon-gamma (IFN-γ) and increased the expression of IFNGR1, a critical receptor for IFN-γ, thereby partially reversing CRC cells' resistance to immune attacks.
Promising Results in Preclinical Models
In mouse model studies, the overexpression of lncRNA 60967.1 not only facilitated immune cell infiltration but also markedly inhibited tumor growth. Notably, this effect was amplified when combined with anti-PD-1 immunotherapy, suggesting a powerful synergistic approach to enhance cancer treatment.
This research opens up exciting avenues for developing novel therapeutic strategies in battling colorectal cancer, ultimately aiming to improve outcomes for patients who currently face grim prognoses.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)