Breakthrough Discovery: Key Enzyme Mechanism Unraveled in Rare Metabolic Disorder
2025-07-08
Author: Wei
A Game-Changing Study on Homocystinuria
An international team of researchers has made a groundbreaking advance in understanding classical homocystinuria, a rare hereditary metabolic disease, as published in The FEBS Journal. This collaborative effort, led by experts at the CIC bioGUNE research center, sheds light on the complexities of this genetic disorder.
The Hidden Threat of Homocysteine
This disorder complicates the body’s ability to eliminate homocysteine, an amino acid that, when elevated, can wreak havoc on vital organs and systems, including the vascular and nervous systems, as well as affecting the eyes and skeletal structure.
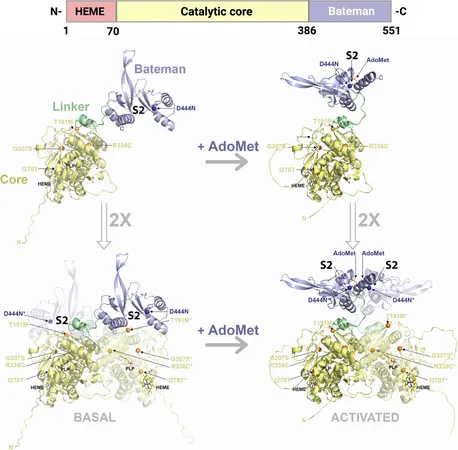
Decoding the R336C Mutation
The research zeroes in on a specific mutation, R336C, impacting the enzyme cystathionine beta-synthase (CBS), crucial for homocysteine metabolism. Surprisingly, rather than destabilizing the enzyme’s structure, this mutation creates an abnormal flexibility that hampers its biological function, leading to toxic amino acid accumulation.
A Chain Reaction of Structural Changes
One of the team’s pivotal discoveries reveals how this mutation triggers a cascade of subtle structural alterations throughout the enzyme. This includes the interaction with pyridoxal phosphate (PLP), a vital cofactor. Instead of reinforcing the enzyme's active form, the mutation disrupts the communication pathway between PLP and the active site of CBS, favoring an inactive configuration.
Impact on Enzyme Mobility and Functionality
The study also notes how the mutation impairs the intrinsic mobility of the Bateman module, a regulatory region of the enzyme. Although the mutated CBS can assemble normally, its motion restrictions hinder substrate access, significantly affecting its catalytic activity.
Opening Doors to New Treatments
In collaboration with experts from the CIBERehd biomedical research network, Qatar University, and the University of Verona, this research paves the way for innovative therapies for those affected by homocystinuria.
Dr. Luis Alfonso Martínez de la Cruz, an associate principal investigator at CIC bioGUNE, emphasizes, "Our study provides one of the few three-dimensional structures of a mutated human CBS enzyme known to date. It explains the catalytic dysfunction of the R336C variant, diverging from earlier assumptions that implicated structural loss in the enzyme. Our findings clarify why patients often fail to respond to vitamin B6 treatment and suggest new therapeutic avenues."
Future Strategies in Personalized Medicine
Potential therapeutic interventions include drug design aimed at restoring communication between CBS and the PLP cofactor, along with personalized therapies to enhance the dynamics of the Bateman module and ensure better substrate access.
This pivotal research not only highlights the importance of international collaboration but also underscores the need for in-depth studies of rare diseases in the quest for tailored and effective medical solutions.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)