Breakthrough Discovery: How Chronic Inflammation Fuels Heart Failure and Potential New Treatments
2024-10-23
Author: Rajesh
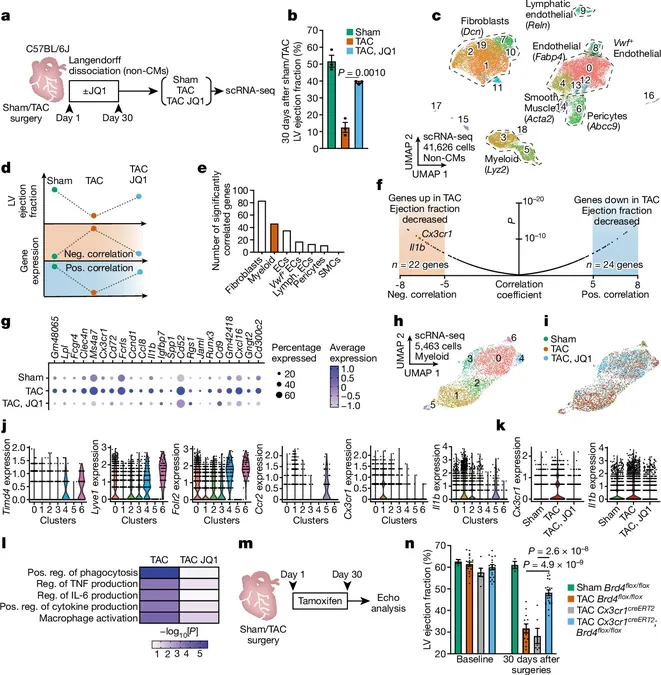
Recent research from the Gladstone Institutes has unveiled a significant link between chronic inflammation and heart failure, revealing how immune cells can activate scar-forming fibroblasts, leading to harmful fibrosis in the heart and potentially other vital organs as well. This groundbreaking study, published in *Nature*, could pave the way for innovative therapies to combat heart disease, a condition that currently affects around 25 million people globally.
When our bodies suffer injuries, such as cuts or surgery, the formation of scar tissue is typically beneficial for healing. However, the relentless buildup of scarring, termed fibrosis, often signifies chronic illness and age-related deterioration in vital organs like the heart, liver, kidneys, and lungs. Unfortunately, treatments for organ fibrosis remain limited and often ineffective, threatening countless lives.
Through their recent investigations, scientists have uncovered the molecular dialogues occurring between immune cells and fibroblasts, the cells responsible for scarring. By obstructing the signals that drive this inflammatory response, researchers showed promise in halting the progression of fibrosis, with some existing drugs already targeting these signals, suggesting a path toward repurposing for heart disease management.
Gladstone Assistant Investigator Michael Alexanian, Ph.D., emphasized the complexity of these interactions, stating, "The crosstalk between the immune system and fibroblasts is even more intricate than we expected. Armed with this new knowledge, we aim to formulate therapies that can simultaneously tackle both inflammation and fibrosis."
Deepak Srivastava, MD, President of Gladstone, highlighted the urgency of this discovery, noting its potential to revolutionize treatment: “Stopping these harmful processes could dramatically improve the lives of millions suffering from heart failure and other fibrotic diseases."
The Heart Disease Inflammation Connection
Inflammation and fibrosis are commonly recognized as simultaneous players in numerous diseases and the aging process. In the heart, the resulting thickening and stiffening of tissues due to fibrosis can lead to critical organ failure. Yet, the exact mechanism by which inflammation triggers fibrosis has remained unclear—until now.
In their study, researchers utilized mouse models to examine how immune cells and fibroblasts change during the progression of heart disease. They administered a BET inhibitor, previously known to reduce fibrosis, aiming to better understand the cellular response to treatment and identify more effective therapies for human patients.
"With this drug, we saw a reduction in both inflammation and fibrosis, as various pro-inflammatory genes were repressed,” reported Arun Padmanabhan, MD, PhD, a cardiologist involved in the research.
Decoding the Molecular Signals
The scientists unraveled a crucial connection between macrophages (a type of immune cell) and fibroblasts, discovering a cascade of events triggered by inflammation. In their previous work, the team identified the gene MEOX1 in fibroblasts, which initiates fibrosis, but the trigger for this activation remained a mystery.
Their current findings revealed that during heart disease, the regulatory gene Brd4 in macrophages becomes active, leading to the production of a signaling protein called IL-1β. This protein then interacts with nearby fibroblasts to activate MEOX1, setting off a chain reaction that exacerbates fibrosis.
"Our insights allow us to target this pivotal sequence of cellular events. By finding intervention points, we can work toward more effective strategies to mitigate fibrosis," elaborated Padmanabhan.
Blocking various elements of this macrophage-fibrosis interaction has shown improvement in heart fibrosis in animal models. Importantly, several drugs aimed at IL-1β are already available for treating other inflammatory diseases, which raises hopes for their applicability in heart disease, though further studies will be necessary to confirm effectiveness in such contexts.
Dr. Alexanian concluded, "By identifying the key signals involved, we can devise novel treatments to combat fibrosis while preserving the immune system's essential role in fighting infections and other diseases."
This major leap in understanding the interplay between inflammation and fibrosis heralds a new era in heart disease treatment, potentially benefiting millions on a global scale.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)