Breakthrough Compound could Revolutionize Glioblastoma Treatment by Targeting the Body's Biological Clock
2025-05-12
Author: Wei Ling
New Hope Against Glioblastoma: The Power of SHP1705
In a groundbreaking development, researchers have unveiled an innovative compound, SHP1705, which specifically targets the circadian clock proteins that glioblastoma stem cells exploit to thrive. This promising compound has not only shown remarkable efficacy in preclinical studies but has also completed a phase 1 clinical trial, demonstrating its safety and tolerability in humans.
Glioblastoma: The Toughest Brain Cancer to Beat
Glioblastoma is the most prevalent malignant brain tumor in adults and poses one of the fiercest challenges in oncology. Current treatments often involve a daunting mix of surgery, radiation, and chemotherapy, yet tumors frequently return with a vengeance and develop resistance to further interventions.
Circadian Clock Proteins: A Target of Opportunity
Circadian clock proteins regulate vital body rhythms, including our sleep-wake cycle. Interestingly, glioblastoma cells hijack these proteins to accelerate their growth. By disrupting this relationship, researchers believe it’s possible to hinder tumor progression.
Steve A. Kay, Ph.D., a leading researcher at the University of Southern California, stated, "We have substantial evidence that cancer stem cells in the brain co-opt clock proteins to fuel their relentless growth. By successfully targeting the circadian clock, we can stifle their replication capabilities."
How SHP1705 Works Its Magic
SHP1705, classified as a CRY activator, enhances the activity of cryptochrome (CRY) proteins that inhibit the circadian clock's machinery in cells. Unlike previous CRY activators, SHP1705 exclusively targets CRY2 proteins, which are abnormally low in glioblastoma cells, making them more susceptible to reactivation. This targeted approach helps shut down the cancer cells' growth mechanisms while sparing healthy brain cells.
Preclinical Success and Clinical Trial Results
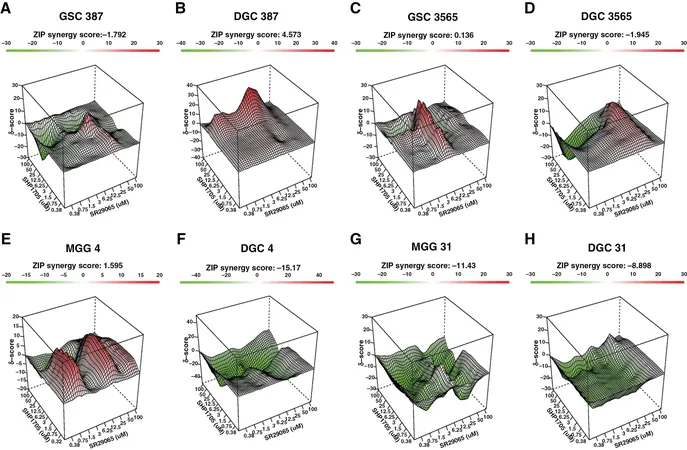
In exhaustive preclinical studies, researchers demonstrated that SHP1705 effectively impaired glioblastoma stem cells' survival. Notably, it proved beneficial against both temozolomide-responsive and resistant stem cell lines. Mouse models further validated its effectiveness, showing that SHP1705 not only slowed tumor growth but also enhanced survival rates.
Additionally, combining SHP1705 with another compound targeting a separate aspect of the circadian clock yielded even more promising results, offering a synergistic approach to treatment.
Next Steps: A Phase 2 Clinical Trial Awaits
After successfully navigating the phase 1 clinical trial with 54 healthy volunteers— where it was deemed well-tolerated with only minor side effects—SHP1705 is set to enter a phase 2 clinical trial. This trial will evaluate its effectiveness alongside traditional treatments such as surgery, chemotherapy, and radiation.
As researcher Chan noted, "The current treatment protocols fail to effectively target glioblastoma stem cells, which are pivotal in the recurrence of this aggressive cancer. We hope SHP1705 can bridge that critical gap and transform the way glioblastoma is treated."
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)