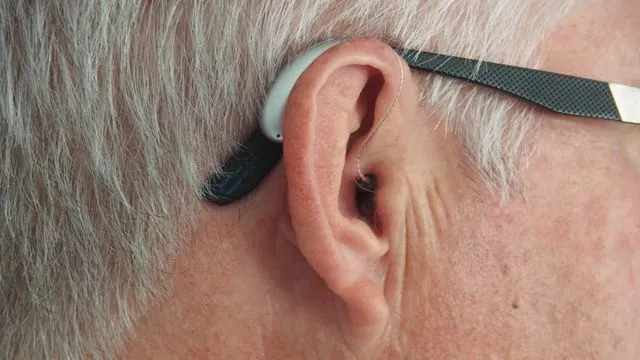
Breakthrough Active Substance AC102 Offers New Hope for Sudden Hearing Loss Treatment!
2024-11-07
Author: Mei
Introduction
In a groundbreaking study led by researchers at MedUni Vienna, a novel active substance named AC102 has shown promising potential for treating acute sudden hearing loss, a condition that affects many people worldwide. The findings, which were featured in the prestigious scientific journal 'Cell Death & Disease,' indicate that AC102 might significantly advance the field of hearing restoration.
Study Overview
The effectiveness of AC102 was tested in an innovative study involving animal models equipped with cochlear implants, conducted by Christoph Arnoldner and Hans Rommelspacher. Currently, cochlear implantation remains the sole treatment option for severe hearing loss, aiming to partially restore hearing ability. However, the surgical procedure risks damaging the inner ear and potentially causing additional hearing loss. Remarkably, the research demonstrated that animals treated with AC102 exhibited significant recovery of their residual hearing compared to those that did not receive the treatment.
Key Findings
Michael Nieratschker, the study's lead author and a member of the Department of Otorhinolaryngology at MedUni Vienna, highlighted the impressive results, stating, 'We observed that the residual hearing in animals administered AC102 improved significantly compared to untreated counterparts.' Further investigation revealed that AC102 possesses anti-inflammatory properties, safeguarding the essential hair cells and auditory nerves from damage, a critical step toward restoring hearing.
Implications for Treatment
The implications of this research extend to treating acute sudden hearing loss, commonly managed with corticosteroids, which recent studies suggest may often fail. Arnoldner points out the dual role of inflammation and cell damage in both sudden hearing loss and cochlear implantation cases, making AC102 a compelling candidate for further research.
Previous Studies and Future Research
Previous preclinical studies have already validated AC102's effectiveness, and a successful Phase I study confirming its safe usage was conducted at MedUni Vienna and Radboud University in Nijmegen, Netherlands. The ongoing Phase II study aims to establish the substance's efficacy in human patients and is being carried out at several European centers, including MedUni Vienna.
Conclusion
With encouraging results so far, both Arnoldner and Nieratschker express optimism about AC102’s potential as a game-changer in the treatment landscape for acute sudden hearing loss. In an era where hearing disorders are prevalent, the ongoing development of AC102 represents a significant step forward. The research could redefine treatment options, offering new hope to those struggling with hearing loss. Stay tuned for updates as this story develops!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)