Are Clinical Trials Keeping Their Promises? A Deep Dive into Data Sharing Practices!
2025-09-01
Author: Wei Ling
Unraveling the Secrets of Clinical Trials: Data Sharing Practices Exposed!
Recent research sheds light on a growing concern in the medical community: the gap between clinical trial registration and the actual data sharing practices. This comprehensive study aimed to investigate how often clinical trials share data as promised, providing insights that could reshape future research standards.
The Study Methodology: What Did They Do?
The researchers focused on the most prestigious medical journals to analyze trials that adhered to the International Committee of Medical Journal Editors (ICMJE) guidelines requiring data sharing plans. By examining trials initiated from January 2019 onward, they filtered through numerous publications to assess their commitment to transparency.
Key Findings: A Staggering Indifference to Data Sharing!
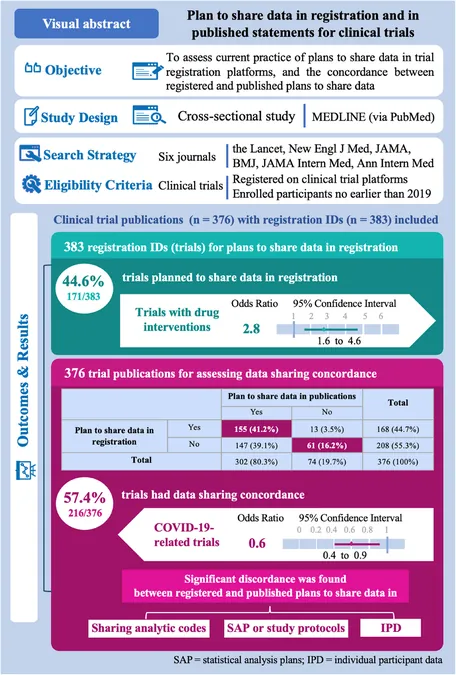
Out of 376 clinical trial publications analyzed, only 44.6% included a clear data sharing plan during registration. Shockingly, over 40% of these trials showed significant discordance between what was registered and what was published, leading to questions about the integrity of research outputs.
Why the Discordance? An Examination of Influencing Factors
The results suggest various influencing factors on data sharing practices. Trials involving drug interventions were more likely to have data sharing plans, while those associated with COVID-19 showed a worrying trend towards decreased concordance. This discrepancy raises alarm bells about the motivations behind data sharing.
The Big Picture: Addressing Clinical Trial Transparency
This study calls for urgent action towards improving data sharing compliance across clinical trials. With over 20% of trials lacking definitive data sharing strategies, there is a pressing need for both educational initiatives and stronger policies to ensure ethical practices in research.
Recommendations for the Future: Enhancing Data Sharing Awareness
The authors stress the importance of structured data sharing guidelines, emphasizing the need to make trial data publicly accessible. Aligning future protocols with recommended best practices can bridge the existing gaps in trial reporting and restore trust in clinical research.
Conclusion: A Call to Action for the Scientific Community!
In a world increasingly driven by data, the findings of this study serve as a wake-up call. It’s time for researchers to honor their data sharing commitments and for journals to hold them accountable. As we continue to navigate the complexities of medical research, enhancing transparency and consistency in data sharing must be our top priority!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)