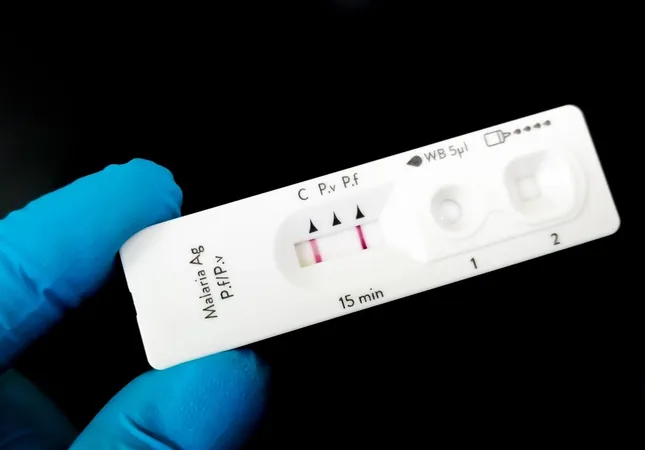
Alarming WHO Alert: Faint Lines on Malaria Tests Could Lead to Misdiagnosis
2025-03-31
Author: Jia
Urgent WHO Alarm on Malaria Test Reliability
The World Health Organization (WHO) has raised an urgent alarm regarding the reliability of rapid diagnostic tests for malaria, particularly after reports of faint positive lines appearing in tests. These faint lines could pose a significant risk of misdiagnosis for patients, potentially leading to severe health complications.
Reports of Faint Lines
Last year, the WHO received multiple reports indicating that these faint lines were predominantly found in patients with low parasitemia – a condition characterized by a minimal presence of malaria parasites in the bloodstream. Surprisingly, even some patients exhibiting higher levels of parasites, who would typically produce clear, strong test lines, displayed these unusual faint results.
Manufacturer Alert
As of now, WHO has not disclosed specific details about the manufacturers involved in this medical product alert issued on March 31. Notably, Abbott, Advy Chemical, and Zephyr Biomedicals are among the brands that reportedly comply with WHO's stringent regulatory standards, according to the agency's product prequalification database. However, exact statistics regarding the most popular brands remain elusive. A study published in the British Medical Journal (BMJ), which surveyed 85,000 participants across various African nations, identified Abbott’s SD Bioline Malaria Ag Pf as the most frequently used rapid diagnostic test.
Consequences of False Negatives
The implications of false negatives in malaria testing are severe; they often lead to misdiagnoses, resulting in delayed treatment and increased patient risks. The WHO emphasized that misdiagnoses could potentially lead to dire outcomes, including death or serious deterioration of health, making the detection of these faint lines critically important.
Importance of Rapid Diagnostic Tests
Rapid diagnostic tests are essential tools in the fight against malaria, especially in areas where lab facilities are scarce. They function by identifying specific antigens produced by malaria parasites circulating in the blood. Depending on the specific test, these diagnostics may target one or several species of the parasite. Their widespread use has significantly boosted testing rates and enhanced malaria case tracking.
Global Impact and Guidelines
Reports on the affected tests indicate errors have been noted in various countries for products detecting both Plasmodium falciparum and Plasmodium vivax, as well as those detecting multiple malaria species. WHO experts have urged those utilizing these tests in national control programs to adhere to proper transportation and storage guidelines, ensure comprehensive training, and encourage visual checks for test clarity. Furthermore, testing locations are advised to report any unusual trends to assist manufacturers in addressing these issues.
WHO Testing Guidelines
WHO guidelines suggest that any visible test line, regardless of its intensity, should be classified as a positive result. Academia has called for advancements in the detection capabilities of these tests, especially considering that in low-light environments, such as those common in some low and middle-income countries, identifying these faint lines can be challenging.
Current Malaria Statistics and Call to Action
With the latest statistics revealing an estimated 263 million malaria cases worldwide in 2023, the gravity of the situation is further underscored. The African region bears the brunt of this health crisis, accounting for 94% of global malaria cases and an alarming 95% of related fatalities. As the fight against malaria continues, the WHO’s findings highlight the urgent need for improvements in diagnostic accuracy to protect vulnerable populations from this deadly disease.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)