A Groundbreaking Study on Inhaled Sedation vs. Propofol for ICU Patients: What You Need to Know
2025-03-31
Author: Jia
Study Design and Ethical Considerations
The study will adhere to ethical standards, obtaining approval from both local and central Institutional Review Boards (IRBs). Participants must be adults on invasive mechanical ventilation, requiring sedation levels ranging from RASS -1 to -4 for more than 12 hours. However, those with neurological conditions, existing sedative needs, or prolonged prior mechanical ventilation will not be eligible.
Informed consent will be collected from either the patients themselves or their legally authorized representatives, ensuring full ethical compliance in the enrollment process.
Training and Implementation
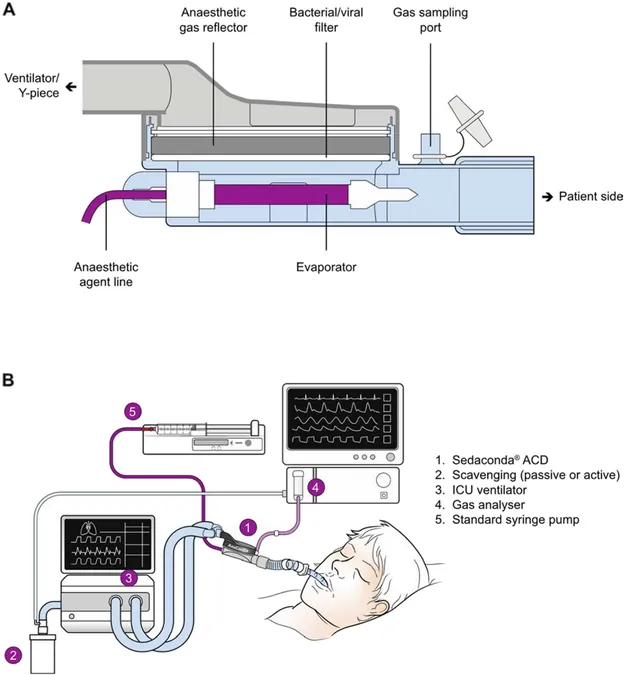
Prior to the commencement of the trial, site researchers will undergo extensive training led by respiratory therapist Clinical Education Specialists. This training will cover isoflurane administration techniques and standardization of assessments, among other critical protocols. Each site will also implement a run-in phase with non-randomized patients to familiarize staff with isoflurane delivery methods via the Anesthesia Conserving Device (ACD-S).
Randomization and Treatment Protocols
Participants will be randomly assigned to either inhaled isoflurane or intravenous propofol using a sophisticated web-based system that ensures a 1.5:1 ratio. This design increases the availability of data on the use of isoflurane for sedation, which the trial uniquely investigates.
Isoflurane will be administered through the ventilator system, requiring adaptations for optimal delivery. The innovative ACD-S device enables efficient anesthetic delivery while minimizing waste and ensures reduced ambient exposure.
Noteworthy Aspects of the Trial
1. **Blinding Mechanisms**: Although a double-blind design poses challenges due to the need for titratable sedation, the study will utilize innovative methods such as sham setups to mask treatment assignments from assessors, allowing for unbiased evaluations of the treatment effects.
2. **Primary Outcomes**: The trial's main goal is to determine the percentage of time patients maintain targeted sedation levels (RASS -1 to -4), which is vital for their recovery and comfort during mechanical ventilation.
3. **Secondary Goals**: The study aims to assess various secondary outcomes, including changes in opioid use, wake-up times following sedation cessation, cognitive recovery, and the proportion of time spontaneous breathing is encouraged.
Safety Monitoring and Data Management
Rigorous monitoring for adverse events will be a continual process throughout the study, managed by an independent Data Safety Monitoring Board (DSMB). This ensures the safety of participants while allowing researchers to gather valuable data on the effects of both sedation methods.
The trial utilizes state-of-the-art electronic data management systems to guarantee the confidentiality of participant information and integrity of collected data, a necessity in contemporary clinical research.
Conclusion: A Potential Shift in Clinical Practice
The INSPiRE-ICU2 trial not only promises to contribute crucial data to the existing body of knowledge surrounding ICU sedation but could also reshape future protocols for managing patients requiring intensive sedation and mechanical ventilation. If successful, the findings may lead to a paradigm shift in how we approach sedation in critical care, paving the way for safer and more effective patient-centered care practices.
Stay tuned for updates on this potentially groundbreaking trial! Your insights could greatly influence the future of patient care.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)