Insurance Denial Puts Six-Year-Old Tennessee Boy's Life on the Line: A Family's Heartbreaking Battle for Gene Therapy
2024-09-16
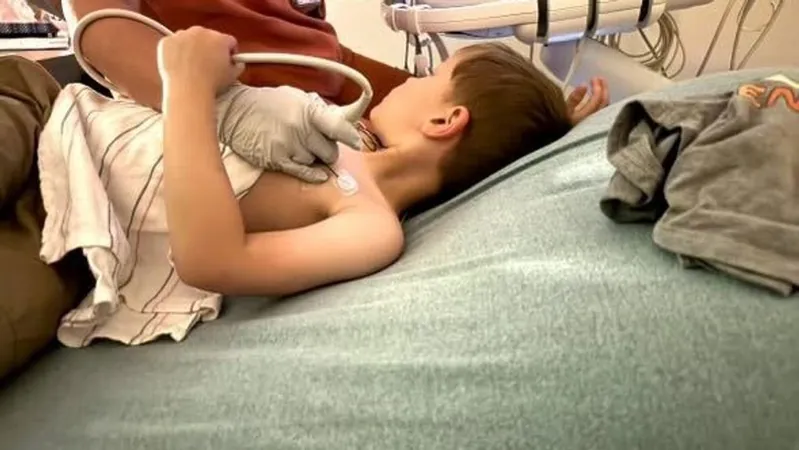
In a heartbreaking turn of events, six-year-old Cooper Wood from Tennessee has been denied life-saving gene therapy by Blue Cross Blue Shield of Michigan (BCBSM), despite being medically approved for the treatment. Cooper was diagnosed with Duchenne muscular dystrophy (DMD) earlier this year, a severe genetic disorder that results in muscle degeneration due to mutations in the dystrophin gene.
Cooper's mother, Kaylin West, expressed her anguish over the insurance company’s swift denial, stating, “Blue Cross Blue Shield is just letting our kids have a death sentence without giving a second thought.” The situation has become dire for Cooper; currently, his only treatment consists of steroids, which merely slow the muscle deterioration caused by this fatal condition.
Ryan Wood, Cooper's father, articulated the family's grim outlook: “In a wheelchair. His ability to walk towards a teenager. Teenage years, his organs will be affected more, eventually, ultimately, most children don't live past their 20s.” The prognosis for children with DMD is indeed alarming, reinforcing how critical timely intervention is.
There is hope, however, in the form of Elevidys, a groundbreaking gene therapy costing $3.2 million. This one-time infusion works by replacing the missing pieces of a vital protein in Cooper's muscles, aiming to repair rather than revert the damage already inflicted. Although it won't restore lost muscle tissue, it could significantly improve the quality of life for many children suffering from DMD.
The Wood family applied for Elevidys on August 20, but by the very next day, their request was denied. Kaylin emphasized, “He deserves more than one day of deliberation.” BCBSM argued that the treatment is considered investigational, citing insufficient data to support its clinical benefits for DMD patients. However, it is crucial to note that the U.S. Food and Drug Administration approved Elevidys for individuals four years and older with a confirmed mutation in June, based on comprehensive studies involving 218 male DMD patients.
“This is a slap in the face, not only to us but most offensively, to the boys with Duchenne muscular dystrophy that spent their entire lives in clinical trials that have led to research like gene therapies,” lamented Kaylin.
In an effort to overturn the insurance's decision, Vanderbilt Hospital initiated an appeal, but as the family awaits results, every day that passes is a day of lost muscle function for Cooper. “Every day is just a little bit more loss of muscles that we need them to keep. We can't sit and wait on this,” Kaylin stressed, emphasizing the urgency of their situation.
When contacted for comment, BCBSM reiterated their position: “There is insufficient evidence that Elevidys provides a clinical benefit in patients with Duchenne muscular dystrophy.”
The Wood family believes that no child should endure such a fight. Ryan Wood passionately remarked, “They all deserve more time, for sure, whether it works or not, they deserve a chance. Insurance companies shouldn't be able to tell us what timeframe we should have for our children.”
In response to this devastating denial, Cooper's parents are actively seeking support and resources. They have initiated an email campaign for individuals to share assistance or similar experiences. Additionally, they are circulating a petition urging BCBSM to reconsider their decision regarding Cooper’s treatment.
As the Wood family battles against time and bureaucracy, their story sheds light on the very real struggles families face within the healthcare system, battling not just illness but the bureaucratic red tape of insurance policies that decide the fate of their children.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)