Breakthrough in Mental Health! F.D.A. Approves Revolutionary New Schizophrenia Drug: Here’s What You Need to Know!
2024-09-27
Introduction
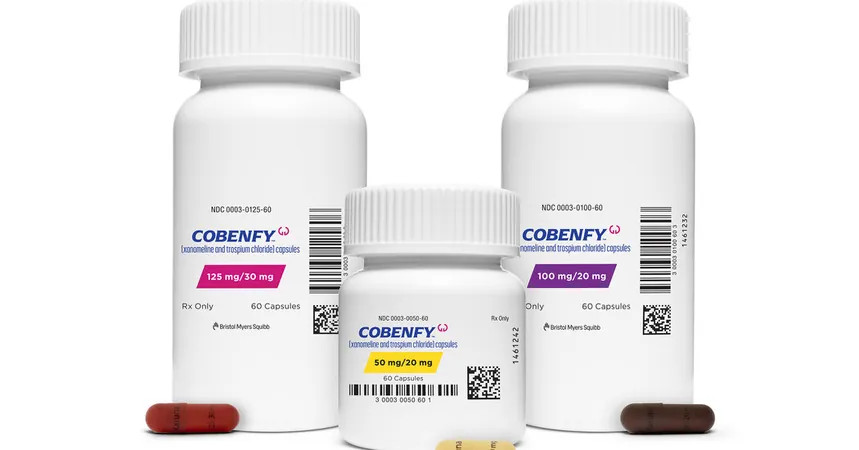
In a groundbreaking move that could change the landscape of mental health treatment, the Food and Drug Administration (F.D.A.) has approved Cobenfy, the first innovative antipsychotic medication in over 70 years, specifically designed to tackle schizophrenia without the debilitating side effects typically associated with existing treatments.
Traditional Antipsychotics
Traditionally, antipsychotic drugs have operated by blocking dopamine receptors in the brain, effectively controlling symptoms such as hallucinations and paranoia. However, these medications have significant drawbacks, including weight gain, which leads to increased risk of cardiac diseases and premature death among patients with schizophrenia. Many individuals have also reported feeling sluggish and unmotivated while on these medications, causing many to stop their use altogether.
Cobenfy’s Unique Approach
Cobenfy aims to address these issues by influencing dopamine levels in a different way—by indirectly altering the levels of acetylcholine, another neurotransmitter in the brain. Researchers are hopeful that this unique approach will alleviate some of the toughest symptoms of schizophrenia, including reduced motivation and the inability to experience pleasure. Dr. Frederick C. Nucifora from Johns Hopkins School of Medicine expressed genuine excitement about Cobenfy, noting that this new mechanism represents a significant advancement in treatment options.
Concerns and Efficacy
Despite the hype surrounding the drug, concerns remain regarding its efficacy over the long term, as only three brief studies—each lasting five weeks—have been conducted. Dr. David Rind, chief medical officer at the Institute for Clinical and Economic Review, stated that healthcare providers and patients are cautious about the drug's effectiveness beyond the initial short-term trials.
Promising Preliminary Results
Promising preliminary results from Bristol Myers Squibb (BMS), the company behind Cobenfy, suggest that patients in extended trials have reported no adverse metabolic changes. Full results are expected later this year, which should provide more clarity on the drug's safety and long-term effects.
Market Impact and Pricing
The approval of Cobenfy is generating a wave of enthusiasm on Wall Street, as analysts predict it could rake in annual sales between $3 billion and $5 billion, especially if it's shown to be beneficial for treating psychosis in dementia patients as well. BMS has priced the drug at approximately $1,850 per month, which aligns closely with other branded antipsychotics. However, this pricing strategy may mean that patients will need to try generic options before being prescribed Cobenfy.
Safety Profile
Another noteworthy aspect of Cobenfy is that it does not carry the F.D.A.'s boxed warning—the strongest alert for patients and practitioners regarding serious side effects—a characteristic that distinguishes it from current antipsychotic options. However, some patients in clinical trials experienced gastrointestinal issues such as nausea and constipation.
Patient Perspectives
Patty Mulcahy, a filmmaker diagnosed with schizophrenia, expressed eagerness to try Cobenfy, stating that her current treatment comes with unwanted side effects that diminish her quality of life. Like many patients, she's looking for alternatives that can restore her energy and joy.
The Need for Better Treatments
While schizophrenia is not prevalent—impacting about 1% to 3% of the adult population—it poses a significant burden, compounded by a lack of robust community care in the U.S. Patients often cycle through jails, hospitals, and homelessness, highlighting the urgent need for effective treatments.
The Importance of Addressing Schizophrenia
Dr. Tiffany Farchione from the F.D.A. emphasized the critical nature of this mental illness, stating that its impacts—often debilitating—can lead to approximately 5% of those affected dying by suicide.
Historical Context
The history of antipsychotic medications began with the introduction of chlorpromazine nearly 70 years ago. Despite years of research—largely focused on genetic variants associated with schizophrenia—no major breakthroughs had emerged until now. With Cobenfy's approval, it appears the field of psychiatric medicine may finally be on the brink of a new era, providing hope for millions who cope with unbearable symptoms.
Conclusion
As Cobenfy takes the spotlight, the mental health community watches with anticipation. Will this new drug revolutionize treatment, or will the unanswered questions continue to loom? Stay tuned as we uncover more about this game-changing development!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)