Urgent Recall: Pregabalin Medication Issues Across Canada
2025-05-03
Author: Noah
Critical Recall Alert!
JAMP Pharma Corporation has issued a swift recall of certain batches of its pregabalin medication, alarming patients and healthcare providers alike.
What’s Wrong?
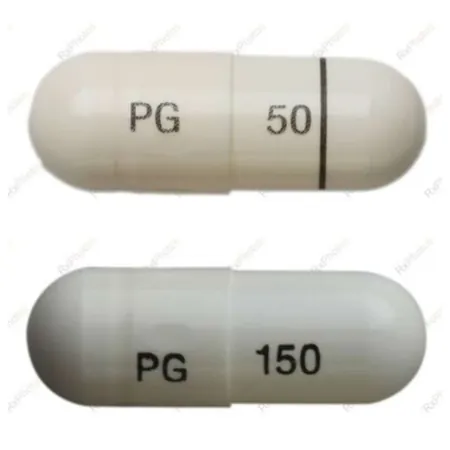
The recall stems from a packaging error where some bottles labeled as containing 50 mg pregabalin capsules might actually contain the stronger 150 mg pills. This could have dangerous implications for patients relying on the medication to manage their health.
How to Check Your Medication
If you have pregabalin at home, it’s essential to check the capsules immediately. Look for the printed number on each capsule—make sure it reads '50' in black ink. If you spot a '150' or have any doubt about your capsules, return the bottle to your pharmacist for a replacement.
Understanding Pregabalin and Associated Risks
Pregabalin is a prescription medication primarily used to alleviate pain from nerve damage caused by conditions like diabetes, shingles, and spinal cord injuries. Additionally, it plays a key role in addressing fibromyalgia pain.
Overdose Risks and Symptoms
It’s crucial to follow prescribed dosages closely—taking too much pregabalin can lead to serious health risks. Symptoms of overdose may include mood swings, excessive sleepiness, confusion, depression, agitation, restlessness, and even seizures. If you experience any of these symptoms, seek medical attention promptly.
Withdrawal Warnings
Moreover, it's important to note that abruptly stopping pregabalin can trigger withdrawal symptoms. Patients might experience insomnia, nausea, headaches, anxiety, excessive sweating, diarrhea, and convulsions. Therefore, any changes to your medication regimen should be done under medical supervision.
Stay Informed and Safe!
Stay vigilant and ensure the safety of your medication. If in doubt or if you're affected by this recall, contact your healthcare provider. Your health and safety come first!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)