Urgent Recall: Eye Drops in Canada Linked to Serious Infection Risks!
2024-09-26
Urgent Recall: Eye Drops in Canada Linked to Serious Infection Risks!
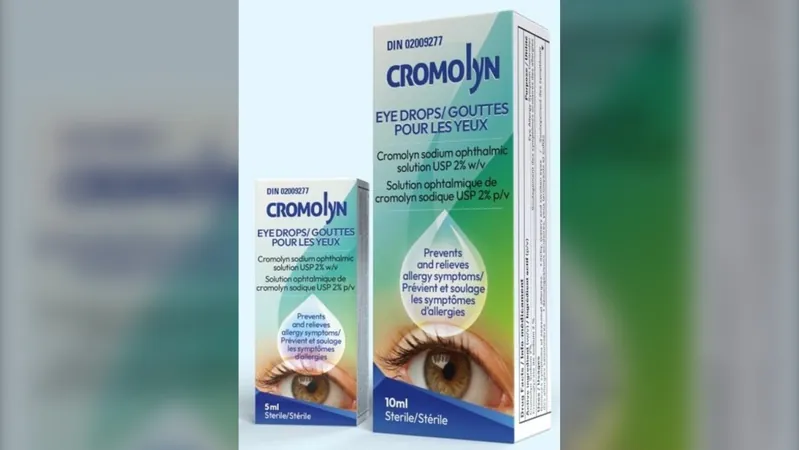
In a startling announcement, Pendopharm, a Montreal-based pharmaceutical company and part of Pharmascience Inc., has issued a critical warning regarding its Cromolyn Eye Drops, raising alarms over potential microbial contamination that could lead to severe eye infections.
Details of the Recall
The recall affects the 5 mL and 10 mL packages of Cromolyn Eye Drops, identified by Drug Identification Number 02009277, with expiry dates ranging from April 30, 2025, to Aug. 31, 2026.
Target Groups at Higher Risk
Health officials emphasize that children, pregnant individuals, seniors, and those with compromised immune systems are at heightened risk for infections resulting from these products.
Advisory and Recommendations
According to an advisory from the Canadian government, although healthy individuals may experience a lower risk of severe infections, the potential is still there, and self-healing may not always occur.
Users are strongly urged to return these eye drops to their local pharmacy for safe disposal to mitigate any risks.
Usage of Cromolyn Eye Drops
The product is commonly used as an over-the-counter remedy for seasonal allergic conjunctivitis—commonly referred to as pink eye.
Concerns Over Preservatives
However, recent testing revealed significant shortcomings in the preservatives used, which are less effective than anticipated.
This inadequacy raises concerns about bacterial growth, particularly that of Pseudomonas aeruginosa, a notorious pathogen linked to severe infections.
Symptoms of Infection
Symptoms of infections could manifest as eye pain, changes in vision, increased sensitivity to light, persistent redness, unusual discharge, and irregular pupil responses.
Potential Consequences
Although infections can often be treated, potential severe cases may lead to life-altering outcomes such as vision loss or systemic infections, particularly for individuals with fragile health conditions including cystic fibrosis, diabetes, or HIV.
Monitoring and Reporting
Health Canada is diligently monitoring the situation, ensuring that Pendopharm implements necessary corrective measures.
They have also pledged to keep the public informed about any emerging health risks.
Contact Information
The company invites individuals who have used these eye drops and have health concerns to consult healthcare professionals.
Concerns or adverse effects can also be reported directly to Health Canada.
Conclusion.
Stay vigilant and contact Pendopharm if you've purchased these eye drops by calling 1-888-550-6060 or emailing [email protected]. Your health could be at risk—act now!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)