Unveiling the Mystery: Why Platinum Electrodes Corrode and What It Means for Green Energy
2025-01-24
Author: Jacob
Unveiling the Mystery: Why Platinum Electrodes Corrode and What It Means for Green Energy
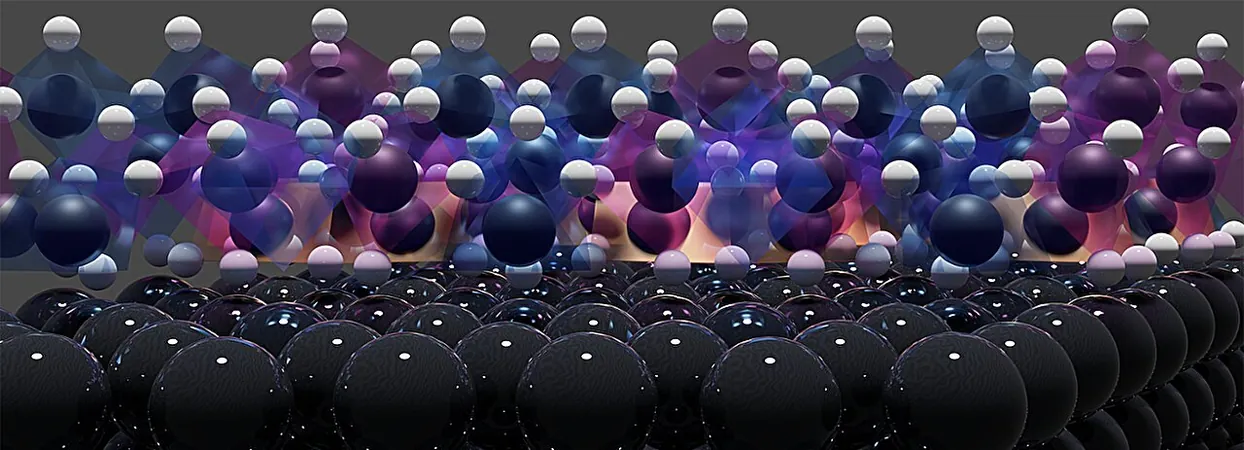
In a groundbreaking study by scientists at Leiden University and the Department of Energy's SLAC National Laboratory, the enigma behind the rapid corrosion of platinum electrodes has finally been unraveled. This pivotal discovery not only promises advancements in more economical green hydrogen production but also enhances the reliability of electrochemical sensors, essential for various technological applications.
Published in the prestigious journal *Nature Materials*, this research tackles a perplexing phenomenon in electrochemical science. Unlike most metals, which are typically protected from corrosion when negatively polarized, platinum electrodes tend to deteriorate rapidly under such conditions. This poses a significant challenge, especially since electrolysis—the process of generating hydrogen from water—relies heavily on negatively polarized platinum electrodes immersed in electrolyte solutions, typically resembling saltwater.
Dimosthenis Sokaras, a senior scientist at SLAC and the principal investigator of the study, pointed out that “being stable doesn’t mean it doesn’t degrade at all,” highlighting the inherent fragility of platinum, despite its well-known durability and high costs.
What Triggers Platinum's Downfall?
The inquiry into the causes of platinum corrosion revealed two leading theories. Many researchers initially suspected that sodium ions from the electrolyte intrude into platinum's atomic structure, forming unstable platinides that eventually detach. Another hypothesis implicated both sodium and hydrogen ions cooperating to create platinum hydrides, resulting in corrosion.
To observe the corrosion process firsthand while the platinum electrodes were operating, the research team harnessed the capabilities of SLAC's Stanford Synchrotron Radiation Lightsource. They utilized high-energy-resolution X-ray spectroscopy techniques capable of penetrating the electrolyte, allowing real-time monitoring of platinum’s deterioration.
Thom Hersbach, a SLAC scientist, emphasized the uniqueness of their approach: “High-energy-resolution X-ray absorption spectroscopy was the only technique that could effectively analyze these complex experimental conditions.”
The Breakthrough: Platinum Hydrides
The extensive research culminated in the first-ever visualization of platinum corroding in real-time, documenting the X-ray spectra emitted from the negatively polarized electrode. The team, which had long suspected the role of hydrides in corrosion, spent years analyzing data to substantiate their theory.
Using advanced computational models, the researchers simulated the expected spectra from both platinum hydrides and platinides. The findings indicated that only platinum hydride could produce the observed results, cementing the team's hypothesis.
“Advancing the frontiers of X-ray science,” Sokaras noted, “coupled with cutting-edge supercomputing, enables us to address scientific inquiries that have lingered for decades.”
Implications for the Future
The outcomes of this study potentially revolutionize the design and operation of electrolyzers and other electrochemical technologies, pushing the boundaries toward more sustainable energy solutions. As scientists continue to collaborate and integrate diverse expertise, as Koper remarked, “it showcases how crucial it is to pool knowledge in the scientific community.”
These findings are not just an academic achievement; they hold immense promise for the future of green energy, signaling a potential shift toward cheaper, more efficient hydrogen production—an essential ingredient in the fight against climate change. Stay tuned as this groundbreaking research paves the way for innovative technologies that promise to reshape our sustainable energy landscape.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)