Unveiling the Hidden Risks of Triptorelin: A Comprehensive Review of Adverse Events
2025-09-01
Author: Olivia
What Is Triptorelin?
Triptorelin, a synthetic analogue of gonadotropin-releasing hormone (GnRH), was first synthesized in the 1970s and boasts enhanced receptor-binding capabilities. This alteration allows for prolonged action in the body, making it a vital player in hormone modulation.
The Power and Purpose of Triptorelin
Used primarily in the treatment of hormone-dependent cancers in men, particularly prostate cancer, Triptorelin drastically lowers testosterone production. In women, it is used to manage estrogen-dependent conditions like endometriosis, and it also helps control precocious puberty in children aged two and older.
Understanding the Side Effects
Despite its effectiveness, Triptorelin is not without risk. Reports of adverse reactions include hot flashes (occurring in over 70% of patients), sexual dysfunction in men, and various gynecological symptoms in women. Pediatric patients may face unique challenges, such as growth changes and allergic reactions. Noteworthy is the generally reversible nature of significant side effects like bone density loss once treatment ceases.
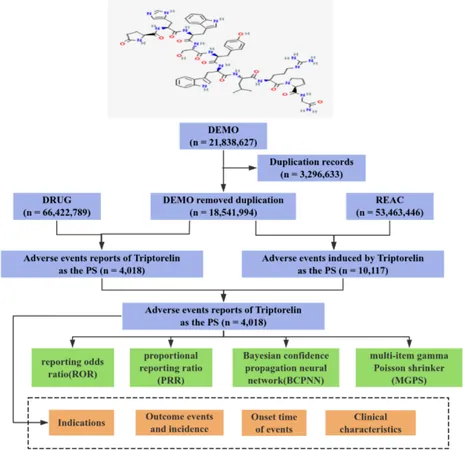
Harnessing Data: The Role of FAERS
The FDA Adverse Event Reporting System (FAERS) serves as a critical tool for ongoing drug safety monitoring. A recent extensive analysis of FAERS data from 2004 to 2024 sheds light on potential safety signals associated with Triptorelin, revealing adverse events that controlled trials may not have highlighted.
What the Data Reveals: A Dive into Adverse Events
A staggering 21 million reports were analyzed, leading to over 10,000 identified adverse events related to Triptorelin. Notably, serious complications such as hospitalization and even death were logged, particularly among older patients battling advanced cancer, emphasizing the need for careful management.
Spotting Patterns: Trends Over Time
Reports of adverse effects peaked notably in 2019, coinciding with increased awareness and medical usage of Triptorelin. Tracing these trends helps to understand the growing landscape of patient experiences and the need for vigilant monitoring.
Decoding Age and Gender Differences in Adverse Events
Analysis revealed distinct trends based on age and gender. Younger patients faced unique side effects like weight changes, while older individuals reported more severe outcomes, indicating the critical importance of tailored treatment plans.
Surprising Signals and Unexpected Findings
The analysis detected several unexpected safety signals, including behavioral changes and serious psychiatric conditions. These findings underscore the need for comprehensive research to explore these potential risks further.
Moving Forward: The Call for Vigilance and Research
While the identification of numerous adverse events is concerning, it is essential to interpret these findings with caution. The data brings forth crucial insights for healthcare professionals to monitor patients closely while signaling the need for further rigorous studies to confirm these associations.
Conclusion: The Ongoing Journey in Pharmacovigilance
As Triptorelin continues to be a cornerstone in managing hormone-related conditions, understanding its safety profile through extensive data analysis is vital. Ongoing vigilance and future research are essential to ensuring patient safety and efficacy in treatment.







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)