Unveiling the Gaps: Revealing the Realities of Data Sharing in Clinical Trials
2025-09-01
Author: Charlotte
A Cross-Sectional Study Exposes Profound Insights into Data Sharing Practices
In an illuminating cross-sectional study, researchers have spotlighted alarming discrepancies in how clinical trials report their data sharing plans, drawing from the strengths and guidelines outlined in the STROBE framework. The study meticulously analyzed data from six of the most prestigious medical journals, unearthing trends that could reshape how we view transparency in medical research.
Search Strategy and Trial Selection: A Methodical Approach
The research team embarked on a thorough selection process, targeting the top ten medical journals based on their impact factor and focusing on clinical trials published from 2019 onwards. This led to a refined list of six journals: The Lancet, The New England Journal of Medicine, JAMA, BMJ, JAMA Internal Medicine, and Annals of Internal Medicine, which collectively hold significant authority in the field.
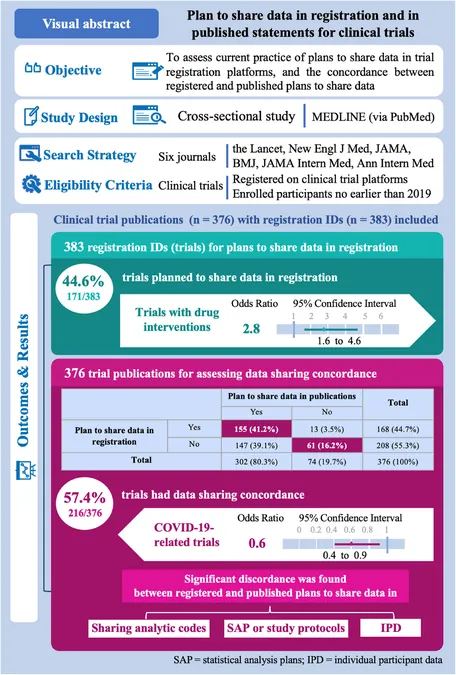
Key Findings: Data Sharing Plans in the Spotlight
Among 376 clinical trials evaluated, a staggering 44.6% reported intentions to share their data, while 34.7% explicitly indicated a refusal. Alarmingly, about 20.6% of trials left their data sharing intentions ambiguous. Notably, a slight upward trend in data sharing plans was observed in 2023 compared to previous years, with a higher prevalence among drug intervention trials.
Concordance or Discordance? A Data Sharing Dichotomy
The study highlighted a significant discrepancy between registered and published data sharing plans. Of the trials assessed, only 57.4% showcased alignment in their data sharing intentions. This discordance, particularly prevalent among COVID-19-related trials, raises pressing questions about the reliability of registered data sharing commitments as researchers navigate the fine line between transparency and competition.
A Call for Improved Data Sharing Practices
The findings underscore a critical need for educational initiatives focusing on the importance of data sharing in clinical trials. It’s evident that many trial authors are either unaware or unsure about how to properly report their data sharing plans, leading to inconsistent and incomplete declarations in publication.
Challenges and Recommendations: Bridging the Gap
Despite existing recommendations, such as those from the International Committee of Medical Journal Editors (ICMJE), issues surrounding privacy, resource allocation, and potential biases continue to obstruct robust data sharing practices. The study advocates for enhanced awareness among journal editors and authors regarding the necessity of complete and accurate data sharing statements.
Conclusion: A Path Forward for Clinical Research Transparency
The study concludes that substantial improvements must be made to bridge the gap between registered and published data sharing plans. The authors recommend a reevaluation of current practices, calling for structured guidance to ensure transparency and reliability in clinical trial reporting. As the pressure for data sharing intensifies, this research shines a light on the critical need for improved practices that ensure all stakeholders can fully benefit from publicly funded clinical research.







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)