Unveiling Nature’s Predator: How a Unique Membrane Protein Powers a Bacterial Nightmare
2025-07-28
Author: Emma
A Revolutionary Discovery in Microbial Warfare
In a groundbreaking study, researchers have unlocked the secrets of a membrane protein that enables a bacterial predator to hunt and devour its microbial competitors. This remarkable discovery not only sheds light on the nature of bacterial predation but also opens new avenues for combating harmful bacteria, with promising implications for medicine and biotechnology.
Meet PopA: The Predator's Secret Weapon
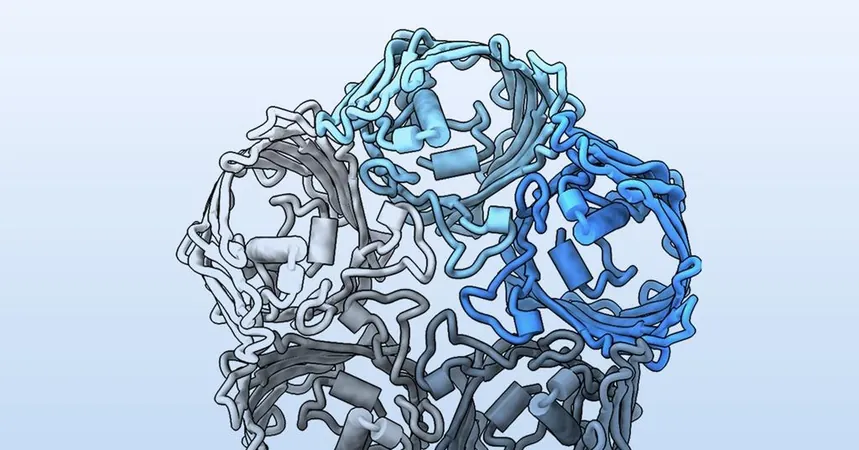
The research centers on PopA, a porin-like protein within the outer membrane of Bdellovibrio bacteriovorus (B. bacteriovorus), a predatory microbe that targets a diverse array of Gram-negative bacteria. Unlike any other known membrane protein, PopA boasts the ability to journey between the outer membrane of its host and penetrate the inner membranes of its prey, challenging longstanding scientific beliefs. "This goes against all conventional rules of how membrane proteins are structured," says Andrew Lovering, a structural biologist from the University of Birmingham, UK, who spearheaded the project.
Unlocking the Mystery: A Fivefold Structure
Lovering and his PhD student, Rebecca Parr, began investigating PopA just before the Covid-19 pandemic hit. Back then, the tools to predict protein structures weren’t as refined, leaving many uncertainties about PopA’s form and function. Through advanced imaging techniques like x-ray crystallography and cryo-electron microscopy, Parr unveiled a unique fivefold structure, a surprising departure from the common tri- or single-part shapes typically seen in outer membrane proteins. A skeptical Lovering recalls his initial doubt, but soon found their discovery left him in awe.
The Predator's Trap: PopA’s Lipid-Capturing Secrets
To delve deeper into PopA’s function, the team introduced it to E. coli, observing significant disruptions in the cells’ membrane integrity. This led to the conclusion that PopA is crucial in how B. bacteriovorus attacks its bacterial prey. Further analysis unveiled that PopA itself has a bowl-shaped structure, capable of ensnaring lipid molecules, thereby disrupting membrane lipid ratios—a tactic that potentially sidelines its target's defenses. Lovering insists this redefines how we view membrane proteins, steering away from the traditional focus on E. coli and Pseudomonas.
Implications for Science and Medicine
Microbiology expert Mohammed Kaplan from the University of Chicago, who did not partake in this research, finds the implications fascinating. "This protein, integral to a predatory bacteria's survival, shifts from its outer membrane to the inner membrane of other bacteria, positioning it as a key player in predation," he explains. This research not only enhances our grasp of evolutionary dynamics but also bridges gaps in understanding cell interactions. However, Kaplan cautions that more questions arise—such as the mechanism through which PopA transitions from predator to prey remains shrouded in mystery.
A New Era in Microbial Research
The study encourages a departure from conventional views in microbiology. As scientists uncover more 'rulebreakers' like PopA in various bacterial species, our understanding of membrane proteins and their potential applications in medical science could be transformed. "This is just the beginning; discovering other unconventional proteins can revolutionize our expectations of what these biomolecules are capable of," Lovering concludes, hinting at an exciting frontier in microbial research.








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)