Unraveling the Timing of Tenofovir Withdrawal: Effects on Hepatitis B and Breastfeeding in New Mothers
2025-08-31
Author: Charlotte
A Global Health Crisis: Hepatitis B in Expectant Mothers
Hepatitis B virus (HBV) remains a significant public health issue, impacting around 240 million people globally. Mother-to-child transmission (MTCT) is a leading risk factor, with approximately 5.5% of women of childbearing age in China testing positive for hepatitis B surface antigen (HBsAg). Despite effective immunoprophylaxis, some newborns still fall victim to chronic hepatitis B from mothers with high viral loads.
Tenofovir: The Go-To Antiviral Treatment
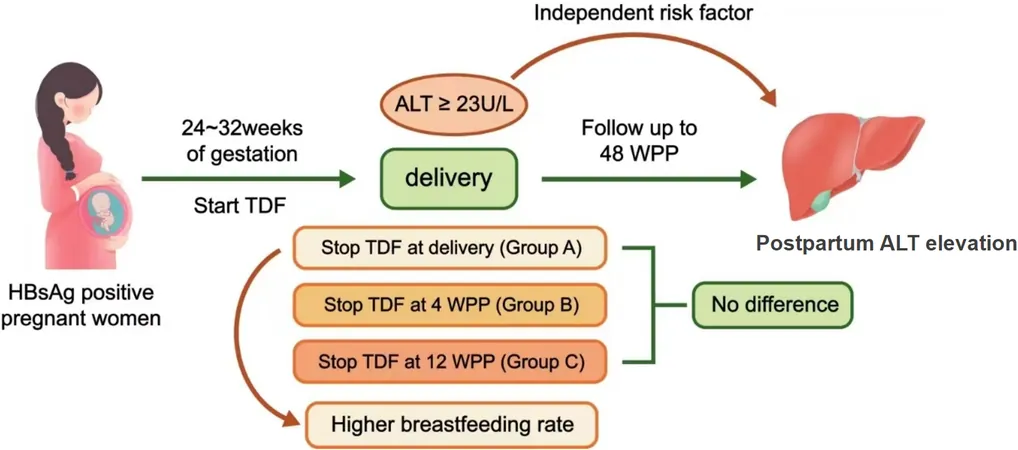
To combat MTCT, antiviral treatments during pregnancy are critical, with Tenofovir disoproxil fumarate (TDF) standing out as a frontline option due to its proven safety and efficacy. The timing of TDF withdrawal postpartum varies significantly across global health guidelines, with some recommending cessation immediately at delivery, while others suggest waiting up to 12 weeks.
Why Timing Matters in Postpartum Care
Understanding the best time to stop TDF is crucial, as prior studies suggest that discontinuing the treatment earlier could lead to increased postpartum elevated alanine aminotransferase (ALT) levels, indicating liver stress. Yet, no comprehensive studies have directly compared the effects of different withdrawal timelines after birth.
A Study to Change the Game: Timing, ALT Levels, and Breastfeeding
In an effort to fill this gap, a recent prospective observational cohort study was conducted to analyze how the withdrawal timing of TDF impacts both elevated ALT levels and breastfeeding rates among mothers on TDF for HBV MTCT prophylaxis. The participating mothers were categorized into three groups based on when they stopped TDF: at delivery, at 4 weeks postpartum, or 12 weeks postpartum. Key findings show that the timing of drug cessation could be key to maternal health and infant nursing.
Significant Findings on ALT Levels and Breastfeeding Rates
The results revealed that a substantial percentage of mothers (over 56%) exhibited elevated ALT levels within the first 48 weeks postpartum, irrespective of their withdrawal timing. However, those who stopped TDF at delivery reported the highest breastfeeding rates at 79%, compared to just 25% and 42% in groups withdrawing at 4 and 12 weeks respectively. This raises vital questions about the influence of perceived drug safety on maternal choices.
Understanding the Implications
The study suggests that early TDF withdrawal corresponds with higher breastfeeding rates, while extending TDF therapy to 12 weeks postpartum helps mitigate the risk of elevated ALT levels in mothers with specific baseline conditions. This insight supports the need for tailored postpartum management strategies that effectively balance maternal liver safety with the promotion of breastfeeding.
Conclusion: A Call for Further Research
This groundbreaking study underscores the complex relationship between antiviral treatment timing and maternal health in breastfeeding mothers with chronic hepatitis B. Future research is necessary to solidify these findings and stress the importance of patient education and informed decision-making in clinical practice.







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)